-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(6): 1960-1963
doi:10.5923/j.ajmms.20251506.72
Received: May 25, 2025; Accepted: Jun. 17, 2025; Published: Jun. 21, 2025

Androgen Receptor as a Pathomorphofunctional Unit of the Sebaceous Gland in Patients with Acne
U. Yu. Sabirov, B. A. Toirov, E. V. Ligay
Republican Specialised Scientific and Practical Medical Center of Dermatovenerology and Cosmetology of the Ministry of Health of the Republic of Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The role of androgens and androgen receptors (ARs) in the pathophysiology of acne represent a complex biochemical process. The article contains data from an immunohistochemical study of patients with acne, which allows us to assess the condition and individual changes in the expression of androgen receptors in the sebaceous gland in accordance with the chosen treatment method.
Keywords: Acne, Androgens, Androgen receptor, Acne treatment
Cite this paper: U. Yu. Sabirov, B. A. Toirov, E. V. Ligay, Androgen Receptor as a Pathomorphofunctional Unit of the Sebaceous Gland in Patients with Acne, American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 1960-1963. doi: 10.5923/j.ajmms.20251506.72.
1. Introduction
- Acne is a multifactorial disease observed predominantly in adolescents and affecting the pilosebaceous unit. It is characterized by hypersecretion of androgens, abnormal keratinization of the pilosebaceous ducts, and secondary bacterial invasion of obstructed glands. Although acne is not life-threatening, it can have serious physical and medical consequences if left untreated. Severe acne can lead to permanent physical scarring, which is considered a risk factor for suicide, especially in men. Other psychological stigmas associated with acne include decreased self-esteem and professional expectations, social inhibition, depression and anxiety [1]. Acne is the single most common skin disorder, affecting 85% of teenage boys and 80% of teenage girls [1]. This disease usually begins in adolescence and often resolves by the mid-twenties [2]. However, there are differences in the presentation of acne among different genders, races, and ethnic groups.The primary site of acne is the face and, to a lesser extent, the back, chest and shoulders. Lesions can be either non-inflammatory or inflammatory. Severe cases of inflammatory acne with large nodules are called nodular cystic or severe nodular acne. Patients may present with hypertrophic scars, especially on the trunk [3].Androgens play a role in the proliferation of keratinocytes and follicular hyperkeratinization of sebaceous follicles, which is observed in acne. Androgens exert their effects through androgen receptors. Androgen receptors (AR) are localized in the pilosebaceous unit, and it has been clinically observed that antiandrogens can shrink follicular casts in these areas [4]. Androgen receptors are present in normal skin, localized in the basal and suprabasal layers of the epidermis and differentiating cells of sebaceous glands [5,6]. Research shows a significant link between testosterone and acne [7]. The most potent androgen is dihydrotestosterone (DHT), which is produced in the sebaceous glands by testosterone 5α-reductase. Local tissue conversion of testosterone to the more potent dihydrotestosterone by the enzyme 5α-reductase has been shown to be increased in the affected skin of acne patients [8,9]. At the cellular level, androgens act by intracellular conversion of testosterone to dihydrotestosterone (DHT) by the enzyme 5α-reductase and subsequent binding of its cytosolic androgen receptor to the nuclear receptor. Binding of the nuclear hormone receptor complex to nuclear chromatin promotes gene expression as a direct response to hormonal stimuli. The degree of hormonal stimulation correlates with the number of androgen receptor binding sites [10]. Androgen receptors on keratinocytes and sebocytes mediate hyperkeratinization, sebaceous gland development, and sebum production [11].Based on earlier studies, it has been suggested that androgen receptors and androgens play different roles in the pathogenesis of acne, and androgen receptors appear to be a better target than androgens for the treatment of acne and other skin diseases [12]. Androgen receptors have affinity for a wide range of steroidal and non-steroidal drugs, and androgen receptor expression has been used to predict clinical response to antiandrogen treatment [13]. An extensive review of the literature indicates the complex role of androgens and androgen receptors in the pathophysiology of acne. The mechanism of action by which isotretinoin exerts its effects on acne-prone skin is also controversial. In addition, there are ethnic and racial differences in the presentation of acne vulgaris. Most of the published work on androgen receptors in acne patients is from Western populations. According to our data, there is no literature on the expression of androgen receptors in the skin of patients with acne in the population of Uzbekistan. To our knowledge, there is virtually no data on the effect of isotretinoin, chlormadinone acetate on androgen receptors in patients with acne in the Uzbek population. Thus, the present study is an attempt to determine the state of androgen receptors in the skin of patients with acne before and after treatment in the population of Uzbekistan.
2. Material and Methods
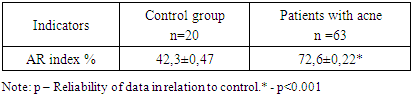
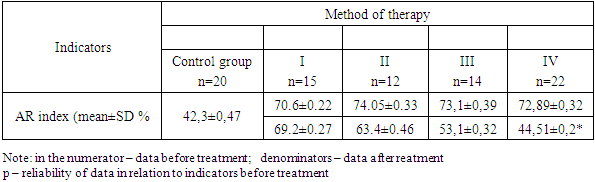
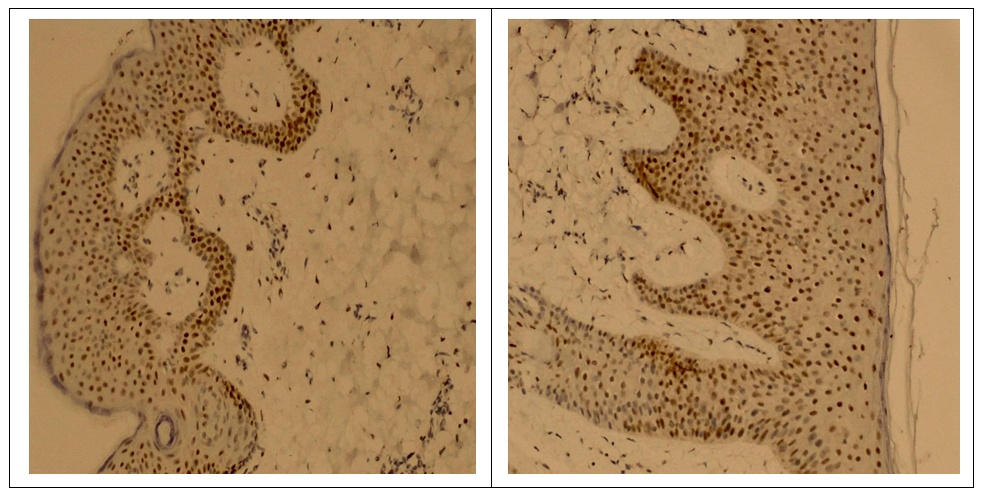
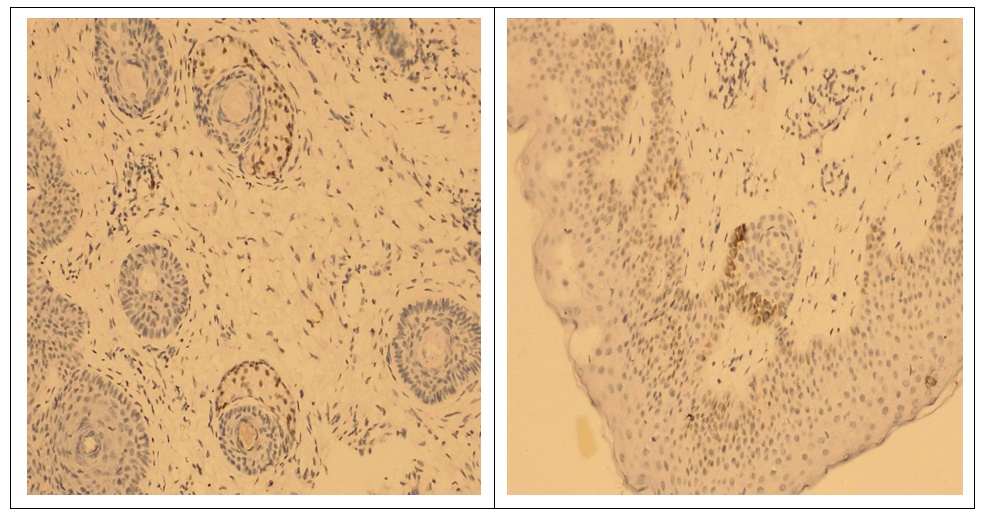
- The study involved 63 female patients aged 19 to 46 years (29.4±3.5 years) diagnosed with moderate to severe acne vulgaris. The selection of patients and the clinical part of the research study were carried out on the basis of the Republican Research and Medical Center of Dermatology and Cosmetology the. Written informed consent was taken from the patients for research, biopsy and subsequent treatment of acne. A detailed medical history was collected from all patients and a complete examination was performed. Before starting treatment, each patient, depending on the intended treatment, underwent the following examinations: general blood test, liver function tests, kidney function tests, lipid and calcium levels in the blood serum, as well as ultrasound of the mammary glands. Patients who had normal test results were included in the study. Patients who planned to have children, pregnant women, and patients with a history of hepatitis were excluded from the study. The patients we observed were divided into 4 groups depending on the treatment methods. The first group included 15 patients with traditional antibiotic treatment; the second group included 12 patients using oral isotretinoin monotherapy; in the third group - 14 patients with systemic monotherapy with chlormadinone acetate; the fourth group included 22 patients with advanced combined treatment with isotretinoin and chlormadinone acetate orally. All patients of all four groups, in addition to the main systemic treatment, were recommended one topical agent and laser therapy with PDL (pulsed dye laser) once a month. After obtaining appropriate approval from the patients, each of them underwent a biopsy at the site of the skin lesion before starting treatment. Needle biopsy was repeated in patients 3-6 months after cessation of treatment. Biopsy samples were fixed in 10% formalin for 24 hours. Paraffin blocks and 5 mm thick sections were made with a rotary microtome. Sections were stained with hematoxylin and eosin.Immunohistochemical test. Thin sections of the paraffin block were cut using a microtome and mounted on poly-L-lysine slides. The sections were deparaffinized and then replaced with acetone (3 times). The slides were then washed and stored in phosphate-buffered saline. The slides were then kept in a 0.03% hydrogen peroxide/methanol block for 30 minutes on a shaker. The peroxide block was then washed three times with phosphate buffered saline. Then an antigen search was carried out. The slides were immersed in prewarmed citrate buffer (pH 6.0) and microwaved for 15–30 minutes and then cooled. After antigen retrieval, this was followed by three washes with phosphate-buffered saline for 5 min each. Slides were then cleared and sections were coated with primary antibodies and kept in a humidified chamber overnight at 4°C, followed by washing three times in phosphate-buffered saline. Sections were coated with secondary antibodies and kept in a humid chamber at room temperature for 20 min. Sections were isolated with tertiary antibodies and kept in a humidified chamber at room temperature for 20 min. The area surrounding the tissue section was cleaned and coated with diaminobenzidine working solution and observed under a microscope to see the appearance of a brown color. The color reaction was stopped by immersing the slide in distilled water. Then the glasses were washed in running tap water. Sections were counter-stained with hematoxylin for 10 seconds to 2 minutes. Then the glasses were washed in running tap water. The sections were dried with acetone for 5 min each (3 times). Sections were cleared with xylene for 5 min each (3 times). The sections were then immersed in D.P.X reagent buffer and the slides were examined under a microscope.Calculation of the androgen receptor index. The androgen receptor index was calculated using the following formula: number of positively stained cells/total number of cells x 100. The mean AR index was calculated for each group and the data were tabulated. Indicators before and after treatment, as well as their comparative analysis (mean ± SD) were summarized in Tables 1 and 2.
3. Results
- The results of immunohistochemical studies are presented in Table 1. As can be seen from the presented data, in patients with acne (the general sample of patients) there is a significant increase in the number of AR index (72.6±0.22% versus 42.3±0.47% in the control at p< 0.001). Our immunohistochemical studies after acne treatment revealed significant differences (P<0.05) between the groups. As the study showed, in group I with traditional treatment there was a positive expression of androgen receptors in the nuclei of gland cells and corresponded to AR-index 69.2 ± 0.27%. In groups II and III, quantitative analysis of nuclei with a positive reaction to androgen receptors revealed results of 63.4±0.46% and 53.1±0.32%, respectively. In patients of group IV with improved treatment, a low level of androgen receptor index was revealed - 44.51 ± 0.20%, which was significantly lower (P < 0.05) compared to patients in groups I, II and III.
|
|
4. Discussion
- Acne vulgaris is a multifactorial dermatological disease observed in all age groups, but mainly in adolescents. It is known that androgens play an important role in the pathogenesis of acne, namely in follicular hyperkeratinization of the sebaceous follicle, which is observed in acne. Androgens cause hypertrophy of the sebaceous glands with increased sebum production. It has been suggested that the development of acne is dependent and influenced by androgens [14]. The action of androgens is mediated by a high-affinity intracellular receptor, the androgen receptor (AR), which is located in the basal and suprabasal layers of the epidermis and the sebaceous glands. An extensive review of the literature revealed the localization of androgen receptors in the skin in the nuclear compartment of the cell [5,15]. However, some researchers report the presence of a positive reaction of androgen receptors both in the cytoplasm and in the nuclear compartment of the cell [16,17]. Cytoplasmic androgen receptors are unbound ARs that translocate into the nucleus only in the presence of its ligand [18]. However, according to some authors, it is believed that bound steroid receptors, such as estrogen receptors, progesterone receptors and androgen receptors, are closely associated with nuclear chromatin, while unbound receptors are more easily displaced from the cell nucleus [18]. Thus, the abundance of steroid receptors in cell cytosol preparations was considered an artifact of cellular fractionation. Therefore, we only considered nuclear androgen receptor positivity, which is the bound form of AR, to calculate the AR index. Studies show that there is a parallelism between AR cytoplasmic positivity and androgen levels [19]. However, in our study, we did not measure serum androgen levels in patients with acne.
5. Сonclusions
- In summary, our study shows that the determination of androgen receptor status in acne patients can be used as a marker for treatment planning for severe cases of acne vulgaris. The results of the study make it possible to evaluate individual changes in the expression of androgen receptors in accordance with the chosen treatment method, which is important in the prognosis and development of effective pathogenetic (antiandrogenic) treatment for acne.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML