-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(6): 1955-1959
doi:10.5923/j.ajmms.20251506.71
Received: May 2, 2025; Accepted: Jun. 16, 2025; Published: Jun. 21, 2025

Comprehensive Clinical and Immunological Analysis of Hemorrhagic Vasculitis in Children
Guloyim S. Avezova, Turdikul A. Bobomuratov, Nafisa S. Sultanova
Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Guloyim S. Avezova, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Hemorrhagic vasculitis is considered one of the most common and frequently occurring diseases in the pediatric population. Its incidence can vary significantly depending on several factors, including geographic region, the level of economic development, and the methods used for diagnosis and statistical reporting. The clinical presentation of hemorrhagic vasculitis is highly diverse, with the severity and potential complications of the disease largely influenced by the state of the hemostatic system—specifically the balance between coagulation and anticoagulation mechanisms. Additionally, the outcome of the disease is strongly affected by the organs involved. In particular, kidney involvement is associated with a notably higher risk of a severe disease course, potentially leading to long-term health issues and requiring more intensive medical intervention. This highlights the importance of early diagnosis, careful monitoring, and a tailored therapeutic approach based on individual patient conditions. The article presents the results of a retrospective analysis of the medical history of 416 children diagnosed with Hemorrhagic Vasculitis who were treated at the Cardiorheumatology Department of the Multidisciplinary Clinic of the Tashkent Medical Academy from 2012 to 2022. Additionally, it includes a prospective study of the clinical and immunological characteristics of the disease in 77 children over time. Key Findings: Hemorrhagic vasculitis most commonly develops after viral infections. The cutaneous-articular form is the most prevalent, accounting for 60% of cases. Inflammatory markers (ESR, CRP) correlate with the severity of symptoms. Glucocorticoid therapy facilitates the rapid regression of symptoms. Patients with this form require long-term monitoring, as 10% may develop chronic complications. These findings provide valuable insights into the clinical and immunological characteristics of hemorrhagic vasculitis, aiding in the optimization of diagnostic and therapeutic approaches.
Keywords: Hemorrhagic vasculitis, Henoch-Schönlein purpura, Clinical features, Children, Immune status
Cite this paper: Guloyim S. Avezova, Turdikul A. Bobomuratov, Nafisa S. Sultanova, Comprehensive Clinical and Immunological Analysis of Hemorrhagic Vasculitis in Children, American Journal of Medicine and Medical Sciences, Vol. 15 No. 6, 2025, pp. 1955-1959. doi: 10.5923/j.ajmms.20251506.71.
1. Introduction
- Hemorrhagic vasculitis (Henoch-Schönlein purpura, IgA vasculitis) is the most common type of primary vasculitis in children. Globally, approximately 3–27 children per 100,000 develop this disease annually. Although relatively rare among all pediatric diseases, it is the most frequently encountered vasculitis in children. The condition typically presents with a hemorrhagic rash (purpura) due to small vessel damage in the skin and has a systemic nature, also affecting the kidneys, joints, and intestines.Hemorrhagic vasculitis mainly occurs in childhood, with approximately 75–90% of cases affecting children under 10 years old. The highest incidence is observed between 4–7 years of age, and it is extremely rare in infancy. In some cases, IgA vasculitis can also occur in adolescence and adulthood, but it is primarily considered a childhood disease [2,4,6,7,9,12].The pathogenesis of hemorrhagic vasculitis is primarily driven by immune-mediated damage to the intimal layer of small blood vessels in various organs, including the skin, joints, gastrointestinal tract, and kidneys. This damage is caused by the deposition of IgA-containing immune complexes, which trigger endothelial dysfunction [1,3,5,6]. Consequently, there is a reduction in fibrinolytic activity, increased activation of lipid peroxidation processes, and significant disruptions in the coagulation and platelet components of the hemostatic system. The significance of this study lies in its aim to identify specific clinical, laboratory, and immunological markers that can be used for the objective and dynamic evaluation of disease activity, thereby contributing to more precise monitoring and improved management of the condition.Research Objective: This study aims to conduct a retrospective analysis of the clinical manifestations and variants of hemorrhagic vasculitis (Henoch-Schönlein purpura) in the pediatric population, with the goal of identifying specific clinical signs, laboratory indicators, and immunological markers that can serve as reliable tools for the objective and dynamic evaluation of disease activity throughout its course. By analyzing these parameters, the study seeks to enhance the understanding of disease progression and support more accurate monitoring and personalized management strategies for affected children.
2. Materials and Methods
- This study was conducted in the pediatric cardiology and rheumatology department of the multidisciplinary clinic at Tashkent Medical Academy. A retrospective analysis was performed on 416 medical histories of children aged 3 to 17 years diagnosed with hemorrhagic vasculitis between 2012 and 2022. Data were recorded in specially designed charts, and children were divided into groups based on age, gender, clinical course, seasonal occurrence, severity, and comorbidities.In order to investigate the clinical and immunological characteristics of hemorrhagic vasculitis over time, a prospective study was carried out involving 77 pediatric patients diagnosed with the condition. The study cohort consisted of 42 boys (54.5%) and 35 girls (45.4%), allowing for a comprehensive assessment of gender-related features and the dynamic progression of the disease within this population. Patients were categorized into three clinical groups based on disease severity:• Group 1 (mild, n=48): skin-joint form.• Group 2 (moderate, n=20): skin-joint-abdominal form.• Group 3 (severe, n=9): mixed form involving skin, joints, abdomen, and kidneys. A control group included 30 age-matched healthy children.Based on disease duration:• 66% experienced acute illness lasting up to 1.5 months,• 26% had a prolonged course lasting up to 6 months,• 8% had chronic relapsing vasculitis lasting over 6 months.Clinical Symptoms. At the onset of the disease:• 14% of patients had a body temperature increase to 37.6–38°C,• 67% experienced loss of appetite,• 34% had nausea and vomiting,• 28% had digestive disturbances (diarrhea or constipation).The diagnostic approach included a comprehensive set of methods aimed at thoroughly evaluating the condition of the patients. These methods encompassed a general clinical examination, complete blood count, biochemical blood analysis, as well as urine and stool analysis. Coagulation tests were performed to assess hemostatic function, and renal ultrasound was conducted to evaluate kidney involvement. Additionally, an immunological assessment of peripheral blood was carried out, which included the measurement of immunoglobulin levels (IgA, IgM, IgG) using Mancini’s method, and the determination of T- and B-lymphocyte levels through the Mendes method. Enzyme-linked immunosorbent assays (ELISA) were also utilized to obtain more detailed immunological data.
3. Results and Discussion
- According to the results obtained, 71.7% of hemorrhagic vasculitis cases in children occurred in the age range of 7-14 years, 2.1% in the 1-3 years group, and 2.8% in the 15-18 years group. Among 416 children, 51.4% ±2.4% were boys and 48.6% ±2.4% were girls, indicating that the disease is more common in boys. This finding corresponds with data from previously studied literature.A seasonal pattern in disease incidence was established, with an increase in cases observed in autumn (September, October, November) and spring (March, April, May). Other researchers have also noted a decline in cases during summer. Notably, Kudryasheva M.A. (2015) justified in her study that in summer, the incidence rate is recorded 3-4 times lower than in other seasons, but severe forms of the disease are more frequently observed in the summer.The duration of hospitalization for children with hemorrhagic vasculitis depends on the clinical form of the disease, its severity, and the presence of comorbidities. According to our results, the average length of hospital stay for children was 10 ±2.3 days.One of the most prominent and clinically significant diagnostic features of hemorrhagic vasculitis in children is the presence of a palpable erythematous rash on the skin. This rash typically serves as a key indicator of the disease and is often one of the earliest and most recognizable symptoms during clinical evaluation. (Pic). Diagnosing the disease before the rash appears is challenging; however, once the rash is present, it confirms the diagnosis. The rash primarily appears on the legs and arms of patients from the 3rd to 4th day of illness.Clinical signs such as acute respiratory infections were frequently observed before the manifestation of hemorrhagic vasculitis symptoms:• Nasopharyngitis - 221 cases (53.1% ±2.4%),• Tonsillitis - 266 cases (23.7% ±2.0%),• Fever - 99 cases (63.9% ±2.3%).These findings emphasize the correlation between upper respiratory tract infections and the onset of hemorrhagic vasculitis in children.Numerous studies investigating hemorrhagic vasculitis in children have highlighted the presence of an allergic predisposition and a history of frequent acute respiratory infections (ARI) as significant contributing factors in the onset and development of the disease. These underlying conditions are believed to play a key role in triggering immune system dysregulation, thereby increasing susceptibility to vascular inflammation characteristic of this pathology. [2,3,4,12,10]. The onset of the disease is often accompanied by nasopharyngeal or intestinal infections, as well as food allergies [11]. The presence of an infectious disease before the onset of hemorrhagic vasculitis (HV) (most commonly a nasopharyngeal or intestinal infection) maintains constant interest in the role of various infectious agents in the development of the disease. According to various data, upper respiratory tract infections occur before the onset of HV in 30-65% of cases [10]. Moreover, several studies have shown a high prevalence of chronic infection foci, particularly chronic sinusitis or tonsillitis, in 74% of children with HV [12].According to the literature, the most common form of the disease is the cutaneous and cutaneous-articular form [2,4,8]. In our observations, all patients predominantly had the cutaneous and cutaneous-articular form as well, with hemorrhagic skin rash observed in 100% of the patients. However, in 93 patients (22.3±2.0%), a normal (cutaneous) form was identified, characterized by purpuric eruptions with a hemorrhagic tint (Fig. 1).
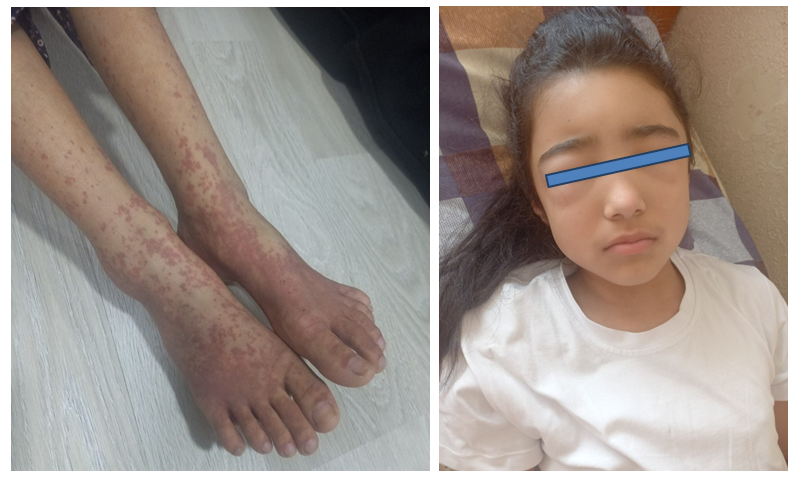 | Figure 1. Severe Recurrent Henoch-Schönlein Purpura (IgA Vasculitis) with Cutaneous-Abdominal and Nephrotic Manifestations in an 12-Year-Old Patient |
4. Conclusions
- 1. Hemorrhagic vasculitis in children presents with diverse clinical manifestations, with cutaneous, articular, gastrointestinal, and renal involvement being the most significant. Hospitalization duration was 2.5–3 times longer in children with comorbidities. Allergic conditions and secondary infections (including helminthic infestations) were identified as predisposing factors for the disease.2. Immunological disturbances in patients included increased CD4+ T-lymphocyte levels and decreased CD8+ T-cells, along with elevated IgA and IgG levels, particularly in mixed forms of the disease. The immune changes observed contribute to the progression of capillary toxicity and prolonged disease course.3. Currently, immunological markers and molecular research are improving early disease detection and helping to determine appropriate treatment strategies. Medical and scientific investigations are playing a key role in understanding the underlying mechanisms of vasculitis and developing more effective treatment approaches.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML