Khayitov Maqsud, Inoyatova Feruza, Muydinov Otabek
Department of Medical and Biological Chemistry, Tashkent, Uzbekistan
Correspondence to: Khayitov Maqsud, Department of Medical and Biological Chemistry, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Liver cirrhosis is a chronic polyethiological progressive liver disease characterized by a significant decrease in the number of functioning hepatocytes, increasing fibrosis, rearrangement of the normal structure of the liver parenchyma and vascular system, the appearance of regeneration nodules, and the subsequent development of liver failure and portal hypertension [3,13,18]. In recent years, cirrhosis and its consequences have remained an important medical and social problem. The disease is characterized by a severe course, reduced quality of life, and increased mortality [3,7,24]. They occupy a major place among the causes of disability in the population and are among the ten most frequent causes of death. According to a major international study of the prevalence of cirrhosis in 195 countries worldwide from 1990–2017, this pathology caused more than 1.32 million deaths worldwide in 2017 (of which 440 thousand were in women and 883 thousand in men), or 2.4% of all deaths [22]. In view of the above, there is a need for more in-depth experimental studies on the molecular mechanisms of liver fibrosis and the development of diagnostic and prognostic criteria for their early diagnosis.
Keywords:
Liver fibrosis, Pathology, Hepatocytes, Parenchyma
Cite this paper: Khayitov Maqsud, Inoyatova Feruza, Muydinov Otabek, Diagnostic Significance of Determination of Intercellular Matrix Components in the Evaluation of Liver Fibrosis Stage in Animals with Experimental Cirrhosis, American Journal of Medicine and Medical Sciences, Vol. 13 No. 3, 2023, pp. 265-270. doi: 10.5923/j.ajmms.20231303.15.
1. Introduction
Currently, liver puncture biopsy (LPB) followed by morphological examination of the punctate is considered to be the gold standard for determining the severity of liver damage [3,15]. Unfortunately, puncture biopsy is an invasive technique with a certain rate of complications, up to and including lethal (according to some studies, the number of lethal cases ranges from 0 to 3.3 per 1000). In addition, the potential of LPB is limited by errors in obtaining the material and in interpreting the results of morphological examination. A number of patients cannot be biopsied because of contraindications. Therefore, a large number of works on noninvasive diagnosis of hepatic fibrosis have been carried out recently, using tests based on methods to detect molecular compounds involved in the pathophysiology of extracellular matrix formation (collagen IV (COL-IV), collagen type III (COL-III) and hyaluronic acid (HA)) [5,8,11,14,17]. However, the data obtained by different authors on the diagnostic value of HA and COL-III and IV for non-invasive screening of liver fibrosis are contradictory and not well understood. Most investigators consider serological markers of fibrosis to be a useful method for monitoring liver fibrosis and determining the stage of fibrosis [4,5,9]. In addition, there is disagreement about the diagnostic value of each individual marker and there are different opinions as to whether a particular marker reflects the degree of inflammation, fibrogenesis activity or the stage of fibrosis that has already developed. When analysing individual markers, we see the greatest disagreement in assessing the diagnostic significance of HA, and the greatest consensus on type IV collagen [11,14,17]. Among direct laboratory markers for the differentiation of fibrosis in chronic hepatitis (CH) and liver cirrhosis (LC), hyaluronic acid (HA) is highly informative: at a concentration above 100 ng/ml, the sensitivity of the test is 100% and the specificity is 84.6% [11].
2. Objective of the Study
To determine the role of N-terminal peptide collagen type III and type IV collagen and hyaluronic acid in estimating the stage of liver fibrosis in animals and the dynamics of liver cirrhosis development.
3. Materials and Methods
Experimental studies were carried out in accordance with "Regulations on the use of laboratory animals in research work and the teaching process of TMA and methods to implement the requirements of biomedical ethics." All experimental animals were kept in the standard conditions of the vivarium, and manipulations with the animals were carried out at the same time of the day. Chronic toxic liver injury with fibrosis or cirrhosis outcome was induced in 35 male rabbits by intraperitoneal injection of 30% CCl4 solution in olive oil at the dose of 1 ml/kg body weight 2 times weekly for 30 days and later 50% solution at the rate of 1 ml/kg 2 times weekly for 50 days. To potentiate the CCl4 hepatotoxic effect, the animals received daily oral doses of 5 ml of 5% ethyl alcohol solution once a day, starting from the 3rd day of the experiment. The duration of the experiment was 60 days. Biochemical and histological examination of the livers of the experimental animals was performed on days 20, 30, 40, 50, and 60 from the start of the experiment. For light microscopy, liver slices were fixed in a 10% formalin solution. Paraffin sections were stained with hematoxylin and eosin (evaluation of general structure) and van Gieson microfuchsin (evaluation of connective tissue), followed by microscopy using Polyvar (Reichert-JUNG, Austria) and Leica DMRE (Leica Microsystems Wetzlar GmbH, Germany) microscopes. A morphological assessment of the fibrosis stage was performed according to the Metavir recommendations [1,20]. The degree of liver damage was assessed biochemically by determining the content of total bilirubin and its fractions, albumin, globulin, urea, and glucose in blood serum; alanine and aspartate aminotransferase (ALAT, AsAT), alkaline phosphatase (ALP), and gamma-glutamyl transpeptidase (GGT) enzyme activity on a biochemical analyzer (Mindray BA-88A, China) using special test systems; and the PTI and platelet count were also assessed. Components of the intercellular matrix (content of hyaluronic acid (HA), collagen IV (COL-IV), and N-terminal peptide collagen type III (PIIINP) in blood serum) were determined by the immunochemiluminescent method on the device MAGLUMI 2000 (Snibe Diagnostic, China) using company biotests. Digital material was processed using the method of variation statistics.
4. Results and Discussion
Studies have shown that lethality of animals was observed mainly in the early experimental period (27%), which was associated with the toxic effects of the combination of CCl4 with ethanol, as according to the literature, ethanol increases the metabolism of CCl4 with the formation of its toxic metabolite. The lethality gradually decreased in the following periods, and at autopsy of the abdominal cavity of the dead animals, the presence of ascitic fluid in the volume of 5–12 ml was noted. Macroscopically, hepatomegaly was noted; the liver was dense, lumpy, and had a fine-grained structure in section. The overall lethality of the experimental group of animals was 32.4%.Morphological examination with assessment of fibrosis according to Metavir showed development in hepatocytes of marked dystrophic, necrobiotic changes and narrowing of sinusoidal spaces due to change of hepatocyte volume with predominance of fat and vacuole dystrophy, expansion of the central vein in the liver on the 20th day of the experiment. In the following terms, they gradually dissolved (30 days), diffuse proliferative inflammatory infiltration developed around both portal tracts and central veins, and their thickening with predominance of histiocytic cells (40 days) (portal tract fibrosis without septum formation, F1) (Fig. 1).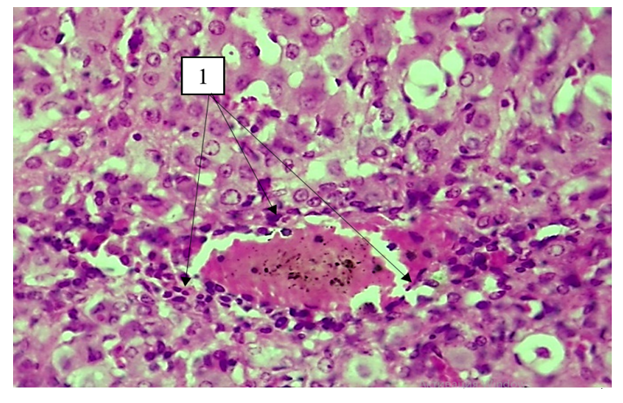 | Figure 1. Day 40 of the experiment. Lympho-histiocytic infiltration around the central vein. Staining: G-E. Uv:10x40 |
Further connective tissue bundles sprouted around the hepatic lobules and reached around the central vein, forming thick and thin bundles of fibrous structures that spread between the cellular elements of the liver parenchyma (50th day) (portal tract fibrosis with single septa, F2) (Fig. 2). 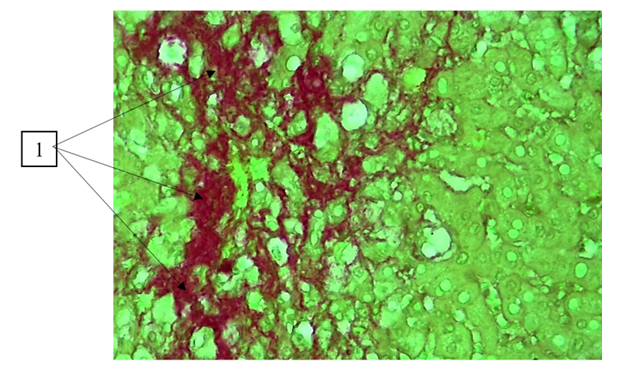 | Figure 2. Day 50 of the experiment. Presence of connective tissue bundles around the portal tracts. Staining: Van Gieson. Uv:10x40 |
By the final term, vacuole dystrophy of hepatocytes, enlarged sinusoidal spaces of varying size, and the presence of bundles of connective tissue around the central vein were detected (Fig. 3).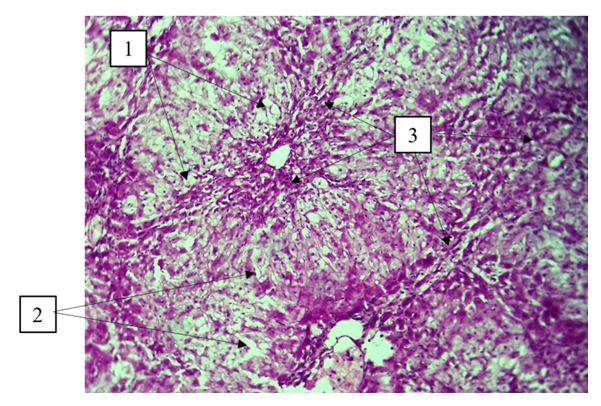 | Figure 3. Day 60 of the experiment. Vacuolar dystrophy of hepatocytes (1), enlargement of sinusoidal spaces of different size (2), presence of connective tissue bundles around the central vein (3). Staining: G-E. Stain: 10x10 |
By this date, densely packed hepatic cells and biliary tubules (fibrosis with numerous septa, F3) were detected within the connective tissue bundles (Fig. 4). It should be said that among the characteristic morphological changes of the liver in this period against the background of fibrosis/cirrhosis were a significant decrease in the ability of cells to reparative regeneration.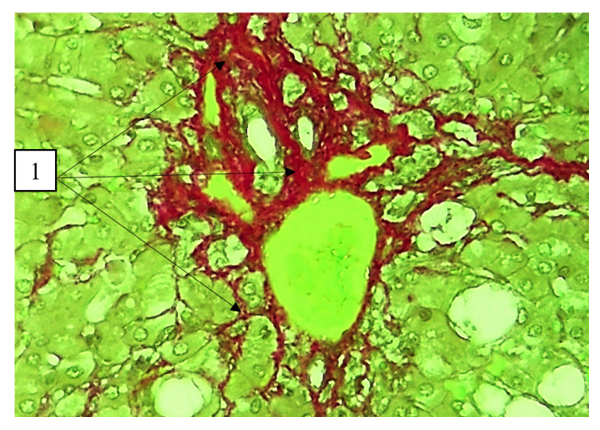 | Figure 4. Day 60 of the experiment. Bundles of collagen fibres around the central vein stained with picrofuchsin. Staining: Van Gieson. Uv: 10x40 |
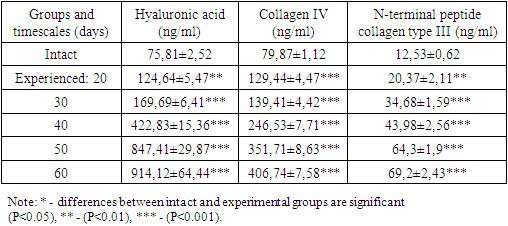
According to the literature, the restoration of parenchymal structure is impeded by the synthesis of type I, III and IV collagens in the perisinusoidal space [19,22]. This leads to impaired microcirculation, organ dysfunction and contributes to portal hypertension. As noted by the authors, the resulting fibrous interlayers cause hepatocyte regeneration in the form of regenerative hepatomas, false lobules, and regenerative nodules [2,19].Direct markers of fibrosis include extracellular matrix metabolites and/or changes in stellate cells that dominate profibrotic cells. These include: HA (a polysaccharide present in the extracellular matrix and elevated in the serum of patients with liver fibrosis), PIIINP (collagen cleavage product), collagens IV and VI, matrix metalloproteinases (a family of enzymes that cleave cellular matrix proteins) [4]. Analysis of these indices in blood serum of experimental animals showed their progressive statistically significant increase with prolongation of the experiment duration (Table 1). Thus, the content of GA increased by 1.64; 2.24; 5.57; 11.18 and 12.6 times, COL-IV by 1.62; 1.74; 3.08; 4.4 and 5.09 times, PIIINP by 1.62; 2.78; 3.51; 3.54 and 3.14 times relative to values of intact rabbits, respectively, at 20, 30, 40, 50 and 60 days from beginning of experiment. The sharp increase in the above-mentioned parameters, especially hyaluronic acid, echoed the development of portal tract fibrosis with single septa and fibrosis with multiple septa by Metavir, which were observed at 40-60 days of experiment.Table 1. Changes in specific direct markers of fibrosis in experimental animals, M±m, n=6-7
 |
| |
|
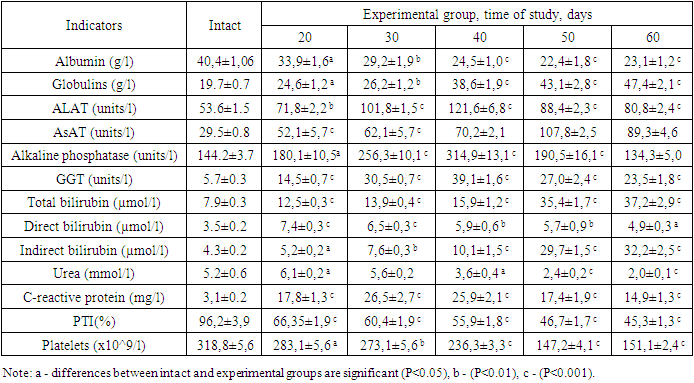
Analyzing the results obtained, it must be said that HA is a polysaccharide of large molecular weight and is widely present in the extracellular space. Part of the produced HA is delivered by the lymphatic system into the venous blood and can be determined in the blood by enzyme immunoassay. In the liver, HA is synthesised by stellate cells and is thought to promote fibrogenesis. Increased HA production may reflect the induction of stellate cell proliferation and the synthesis of extracellular matrix components induced by inflammation [6,12]. The more than 5-fold increase in HA content in blood serum at day 40 is apparently associated with the beginning of fibrogenesis in the liver by stellate cells and intensive production of transforming fibroblast-beta. At the same time, an increase in HA content of more than 10 times on the 50th or 60th day of the experiment is connected with the formation of multiple septa in the liver.This is also proved by the 4-5-fold increase of COL-IV content in the serum of experimental animals on the 50th–60th day of the experiment. It should be said that collagen is an extracellular protein synthesized by stellate cells of the liver as a precursor, which undergoes post-translational changes to form collagen molecules and then fibrils in the extracellular matrix. The formation of covalent intermolecular cross-links provides additional mechanical strength to collagen fibers. Numerous studies have shown an increase in these mature bonds in liver cirrhosis than in normal liver tissue [18,24]. Considering that HA degradation occurs in hepatic sinusoidal and endothelial cells involving specific receptors. On their surface, they contain sieve-like plates with highly specific receptors for HAs, which capture it and undergo degradation under the action of hyaluronidase. Therefore, it is the ratio of the synthesis and degradation processes that determines the HA content in the blood. Probably, a decrease in this process under prolonged exposure to damaging factors in the liver leads to a slowdown of HA degradation and its pronounced increase in the blood. Apparently, the imbalance of synthesis and decomposition processes of HAs allowed to refer it to the direct specific markers of liver fibrosis [11,17].Of equal importance in the diagnosis of liver fibrosis and cirrhosis is a marker such as N-terminal procollagen peptide III, which is the product of collagen breakdown [5,16]. In fibrotic diseases, extracellular matrix turnover is increased, and therefore the activity of mechanisms required for proper collagen processing increases. PIIINP is significantly correlated with necroinflammatory processes in the liver and reflects more the degree of inflammation than the stage of fibrosis [10,16]. Apparently, the absence of an increase in PIIINP level in the serum of experimental animals on days 40–60 of the experiment is associated with some decrease in the necroso-inflammatory processes of the rabbits' liver.Along with this, there were studied indexes of liver damage syndromes: liver cellular failure (platelet, PTI, albumin, and urea content); cytolysis (ALAT, AST activity, bilirubin and its fractions); cholestasis (GGT activity, ALP); and mesenchymal inflammation (globulin content and thymol assay) (Table 2). The studies showed a progressive decline in the synthetic and decontaminating functions of the liver, which was manifested by decreased albumin, urea, and PTI. Increased cytolysis, cholestasis, and mesenchymal inflammation were noted, indicating a toxic effect of CCl4 and ethanol on the liver.Table 2. Changes in specific direct markers of fibrosis in experimental animals, M±m, n=6-7
 |
| |
|
The next stage of our research was to assess the diagnostic value of direct and indirect tests for liver fibrosis. For this purpose, we used the standard APRI test proposed by Yilmaz Y. et al. (2011) [23]. It is calculated as follows:APRI = (patient's AST/(upper limit of AST))100/platelets (10^9/l).If the index value is greater than 1.0, the probability of significant fibrosis is high; if it is less than 0.5, the absence of a significant effect of therapy is highly probable. Indeed, studies have shown that the APRI index in the dynamics of chronic liver damage increases from 0,60±0,06 on the 20th day of the experiment to 0,75±0,06 on the 30th day; 0,98±0,03 on the 40th; 2,42±0,07 on the 50th; and 1,95±0,1 on the 60th day of the experiment. However, this figure is calculated on the basis of the ratio of ASAT activity in patients to the normative values for platelets. Considering that AST activity and platelet count can vary markedly in other pathologies, it was of interest to use liver-specific indexes. Taking into consideration that the leading role in fibrosis development belongs to stellate cell activation, while a decrease in hepatocytes leads to impairment of synthetic, detoxicating liver functions, to our mind it is more reasonable to use the ratio of stellate cell activation parameters (fibrosis processes) to indices of liver cellular failure. Specifically, we calculated the ratio (COL-IV+PIIINP+HA)/(albumin+urea+PTI). This ratio progressively increased to 2.58±0.09; 3.62±0.13; 8.50±0.24; 19.5±1.23; and 18.03±0.76, with a value of 1.19±0.04 in intact rabbits. As seen from the presented results, the abrupt increase of the fibrogenesis index on the background of inhibition of synthetic and decontaminating liver function leads to a progressive increase of this index, especially on the 50th or 60th day of the experiment, when histologically single and multiple septa development is observed. In our opinion, an increase in this index over 5,00 can indicate the beginning of collagen bundle formation (stage F1), over 5,00 to 10,00 - stage F2, 11–15 - stage F3, and over 15, - stage F4 (cirrhosis). Considering that it is economically expensive to determine collagen formation indices simultaneously, we calculated the HA/urea ratio, as according to our data, more pronounced changes are typical for HA, while the level of urea reflects the synthetic and decontaminating functions of the liver. This ratio also increased progressively to 20.5±0.98; 30.12±0.87; 122.34±12.01; 382.7±35.15; and 433.8±34.56, with a value of 15.1±1.58 in intact rabbits. As seen from the given data, it reflects the degree of fibrosis more distinctly: the exceeding of this index over 30,00 may indicate the beginning of collagen bundle formation (stage F1), from 31 to 100,0 - stage F2, 111-200 - stage F3, and over 200 - stage F4 (cirrhosis). It must be said that clinical studies are needed to confirm this assumption, which is the subject of our further research.
5. Conclusions
1) The combined model of liver fibrosis leads to the development of F2 and F3 fibrosis according to the Metavir classification by 40–60 days;2) A progressive increase in intercellular matrix components (COL-IV, PIIINP, and HA) as liver fibrosis is induced by Metavir was observed;3) The calculation (COL-IV+PIIINP+HA) / (albumin+urea+PTI) and a simplified model of HA/serum urea content were proposed to estimate the degree of hepatic fibrosis.
ACKNOWLEDGEMENTS
The authors are deeply grateful to D.S.Allaberganov, associate professor of the department of pathological anatomy of TMA for the help in histological study and its interpretation and to the staff of "NEW INNAVATION PHARM GROUP" Ltd. For help in carrying out these researches.
Conflict of Interest
The authors have no conflicts of interest to declare. We are grateful for the support of Vitros Diagnostics, which kindly provided the kits used for the experiments used in this study.
References
| [1] | Andreev V. P., Tsyrkunov V. M., Kondratovich I. A. MORPHOLOGICAL MONITORING OF EXPERIMENTAL LIVER FIBROSIS IN RATS // Hepatology and gastroenterology. - 2021. - V. 5. - No. 2. - S. 150-160. |
| [2] | Garelik P. V., Mogilevets E. V. Photodynamic therapy for liver cirrhosis (experimental study) // Annals of surgical hepatology. - 2018. - T. 20. - No. 4. - S. 17-26. |
| [3] | Garipov R. M., Tagirova L. R. Cirrhosis of the liver: aspects of pathogenesis, diagnosis and treatment // Clinical and experimental surgery. Electronic scientific and practical journal. - 2014. - T. 13. - No. 3. - S. 1-6. |
| [4] | Didenko V. I. Modern methods for determining liver fibrosis // Gastroenterology. - 2013. - T. 48. - No. 2. - S. 28-35. |
| [5] | Kulebina E. A., Surkov A. N. Progress in non-invasive diagnosis of liver fibrosis: a review of modern laboratory techniques // Medical Council. – 2020. – no. 11. - S. 215-223. |
| [6] | Lebedeva E. I., Myadelets O. D. Cellular and molecular mechanisms of liver fibrogenesis // Hepatology and gastroenterology. - 2019. - Vol. 3. - No. 2. - S. 119-126. |
| [7] | Lyzikov A. N., Skuratov A. G., Prizentsov A. A. Modern surgery for portal hypertension: from classics to innovative technologies // Problems of health and ecology. – 2014. – no. 1 (39). - S. 56-62. |
| [8] | Soloviev Yu. MK Ammosov. Series: Medical Sciences. – 2020. – no. 4 (21). - P. 45-54. Abe S. et al. High-dose interferon-α therapy lowers the levels of serum fibrogenesis markers over 5 years in chronic hepatitis C //Hepatology Research. – 2003. – Т. 25. – №. 1. – С. 22-31. |
| [9] | Dirchwolf M., Ruf A. E. Role of systemic inflammation in cirrhosis: From pathogenesis to prognosis // World journal of hepatology. – 2015. – Т. 7. – №. 16. – С. 1974. |
| [10] | Grzeszczuk A., Chlabicz S., Panasiuk A. Serum hyaluronan as a possible biomarker of liver insufficiency in cirrhotic patients // Roczniki Akademii Medycznej w Bialymstoku (1995). – 2002. – Т. 47. – С. 80-85. |
| [11] | Hou W., Syn W. K. Role of metabolism in hepatic stellate cell activation and fibrogenesis // Frontiers in cell and developmental biology. – 2018. – Т. 6. – С. 150. |
| [12] | Kaur N. et al. Potential role of noninvasive biomarkers during liver fibrosis // World journal of hepatology. – 2021. – Т. 13. – №. 12. – С. 1919. |
| [13] | Koroglu G., Demir K., Ozdil S. et al. Correlation between lever fibrosis stage and serum hyaluronat levels in patients with chronic viral hepatitis // Citation: Gut. -2003. №52 (Suppl VI). - A103. |
| [14] | Martínez-Esparza M. et al. Inflammatory status in human hepatic cirrhosis // World journal of gastroenterology. – 2015. – Т. 21. – №. 41. – С. 11522. |
| [15] | Nøjgaard C. et al. Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease // Journal of hepatology. – 2003. – Т. 39. – №. 2. – С. 179-186. |
| [16] | Patel K. et al. Clinical use of hyaluronic acid as a predictor of fibrosis change in hepatitis C // Journal of gastroenterology and hepatology. – 2003. – Т. 18. – №. 3. – С. 253-257. |
| [17] | Roehlen N., Crouchet E., Baumert T. F. Liver fibrosis: mechanistic concepts and therapeutic perspectives // Cells. – 2020. – Т. 9. – №. 4. – С. 875. |
| [18] | Saffioti F., Pinzani M. Development and regression of cirrhosis // Digestive Diseases. – 2016. – Т. 34. – №. 4. – С. 374-381. |
| [19] | Shiha G., Zalata K. Ishak versus METAVIR: terminology, convertibility and correlation with laboratory changes in chronic hepatitis C // Liver Biopsy. – 2011. – Т. 10. – С. 155-170. |
| [20] | Тhe Lancet Gastroenterology & Hepatology, 2020, V. 5, P. 245-26. |
| [21] | Xu B. et al. Capillarization of hepatic sinusoid by liver endothelial cell-reactive autoantibodies in patients with cirrhosis and chronic hepatitis // The American journal of pathology. – 2003. – Т. 163. – №. 4. – С. 1275-1289. |
| [22] | Yilmaz Y. et al. Noninvasive assessment of liver fibrosis with the aspartate transaminase to platelet ratio index (APRI): Usefulness in patients with chronic liver disease: APRI in chronic liver disease // Hepatitis monthly. – 2011. – Т. 11. – №. 2. – С. 103. |
| [23] | Zhou W. C., Zhang Q. B., Qiao L. Pathogenesis of liver cirrhosis // World journal of gastroenterology: WJG. – 2014. – Т. 20. – №. 23. – С. 7312. |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML

