-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2022; 12(5): 471-480
doi:10.5923/j.ajmms.20221205.05
Received: April 18, 2022; Accepted: May 6, 2022; Published: May 10, 2022

Complex Treatment of Experimental Model of Diabetic Foot Syndrome
Ergashev U. Y., Ernazarov Kh. I., Zohirov A. R., Alzabni I. D.
Tashkent Medical Academy, General Surgery Department №2, Tashkent, Uzbekistan
Correspondence to: Ernazarov Kh. I., Tashkent Medical Academy, General Surgery Department №2, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study was tolearn of the effect of the new drug Reomannisol on wound healing, taking into account pathomorphological aspects in the complex treatment of experimental diabetic foot syndrome. Material and methods. An experimental study was carried out on 140 outbred male rats weighing 220-250 g, kept in the TMA vivarium. The experimental animals were divided into 4 groups: the 1st group was intact; 2nd group – the creation of an experimental model of alloxan diabetes mellitus; 3rd control group - against the background of alloxan diabetes, the creation of an experimental model of a diabetic foot using traditional complex treatment; 4th experimental group - on an experimental model of diabetic foot - traditional treatment and reomannisol. On the skin of each rat’s right hind paw’s footpad, a full-thickness rectangular wound measuring 2 mm×5 mm was created with a scalpel. Every day, the wounds were treated with the traditional method of treatment (5% alcohol solution of iodine and levomekol ointment) until the end of the experiment, for the experimental group, the local traditional method of treatment and intraperitoneal administration of the new Reomannisol preparation were used 1 time per day at a dose of 1 ml of Reomannisol per 100 g of animal body weight for 5 days. Results. By the 10th day in the experimental group, an independent almost complete closure of the wound defect and hair growth around the wound was noted. In the control group, on the 10th day, a wound defect of about 4.9 ± 0.05 mm2 remained, the wound healed on the 14th day of the experiment. Conclusion. The use of local treatment and reomannisol can enhance angiogenesis in the early stages of the experiment and restore disturbed microcirculation, increase macrophage response, fibroblast proliferation, maturation and remodeling of granulation tissue and its epithelization, reduce the inflammatory reaction, which leads to more effective and early healing of the wound area.
Keywords: Experimental model of diabetic foot, Experimental animals, Diabetes mellitus, Alloxan, Surgical debridement, Reomannisol
Cite this paper: Ergashev U. Y., Ernazarov Kh. I., Zohirov A. R., Alzabni I. D., Complex Treatment of Experimental Model of Diabetic Foot Syndrome, American Journal of Medicine and Medical Sciences, Vol. 12 No. 5, 2022, pp. 471-480. doi: 10.5923/j.ajmms.20221205.05.
Article Outline
1. Introduction
- Currently, one of the leading places in terms of growth rates of morbidity, disability, and mortality was occupied by diabetes mellitus (DM) among the so-called "diseases of civilization" [3]. Today, over 460 million people are sufferingglobally from diabetes; according to the predicted facts announced by the International Diabetes Federation, by 2040 the number of patients will increase up to 642 million [11].Diabetes mellitus is accompanied by the development of complications, including diabetic foot syndrome (DFS), one of the leading clinical symptoms of which is the persistence of an ulcer on the skin of the lower extremities [14].Delayed wound healing is one of the complications of the disease due to multiple factors including poor circulation [13], prolonged inflammation, and hyperglycemia. It is a common cause of morbidity and mortality in patients with DM [18]. When the wound becomes chronic, it is prone to developing foot ulcers, including neuropathy and foot deformities [13]. Foot ulcers in DM are the cause of more than 50% of all non-traumatic leg amputations [22]. Evidence has shown that hyperglycemia is one of the main factors contributing to slow wound healing [18] by increasing cell apoptosis and decreasing cell survival in diabetic wounds. It has been shown to inhibit endothelial cell and fibroblast proliferation in humans [23], up to 75% slower in adult mice with DM compared to control mice [19].At the present stage in experimental diabetology, the most widespread chemical model of diabetes mellitus uses substances that destroy β-cells of the islets of Langerhans [12,15]. This study describes a model of diabetes mellitus in rats, induced by the introduction of a reduced dose of alloxan, which significantly reduces the number of animal deaths.The alloxan model of diabetes mellitus is one of the most widespread and studied. It is actively used by researchers around the world. Alloxan is a structural analog of glucose, due to which it accumulates in pancreatic β-cells and leads to their death, followed by the development of diabetes. At the same time, damage to β-cells is accompanied by degenerative changes in the kidneys and liver, which leads to high mortality in laboratory animals on the first day after alloxan administration. The problem of violations of several types of metabolism with the introduction of alloxan, the prevalence of manifestations of oxidative stress as a typical pathological process in case of damage to the key organ involved in all types of metabolic processes (liver) dictate the need to prescribe pathogenetic drugs from the group of metabolic correctors with hepatoprotective and antioxidant orientation. One of the promising new drugs in this area is Rheomannisol (LLC "REKA-MED FARM" Republic of Uzbekistan) - a complex drug with antihypoxic, antioxidant, rheological, anti-shock, detoxifying, diuretic action. The main pharmacologically active substances are sodium succinate and mannitol.
2. Aim of the Study
- Study of the effect of the new drug "Rheomannisol" on endogenous intoxication and wound healing, taking morphological aspects into the complex treatment of experimental diabetic foot syndrome.
3. Materials and Research Methods
- The work was done on experimental material. Healthy rats were selected for the experiment. Experimental studies were carried out on 140 outbred male rats weighing 220-250 g, kept in the Tashkent Medical Academy (TMA) vivarium. The rats were kept under optimal conditions, all rats lived in a room with a 12-hour light-dark cycle and a constant temperature of 22-25°C, with free access to water. All rats were given a sufficient amount of a normal rodent diet ad libitum. (diet for rodents, State standard No. GOST R50258–92) and tap water daily. Operations and all manipulations with animals were carried out using general anesthesia, in compliance with the principles of humanity outlined in the directives of the European Community (86/609/EEC) and the Declaration of Helsinki, by the "Rules for working with experimental animals". The experimental animals were divided into 4 groups: the 1st group was intact; 2nd group –the creation of an experimental model of alloxan diabetes mellitus; 3rd control group - against the background of alloxan diabetes, the creation of an experimental model of a diabetic foot using traditional complex treatment; 4th experimental group - on an experimental model of diabetic foot - traditional treatment and reomannisol.After a 24-hour fast, the rats were weighed. A 2% solution of alloxan diluted in 0.9% saline was administered intraperitoneally as a single dose, corresponding to a dose of 20, 15, 12 mg of alloxan per 100 g of animal weight. Food and water were given to animals only 30 minutes after drug administration. On the 3rd day, the level of glucose in the blood was assessed.Determination of glucose concentration in the peripheral blood of animals. Diabetes was confirmed 3 days after the blood glucose concentration was determined. Peripheral blood glucose concentration was measured with an “AccuChek Active” glucometer (Roche Diagnostics, Germany), the linear measurement range was 0.6–33.3 mmol/L. Blood sampling to study the level of glycemia was performed from an incision in the tip of the tail. An experimental model of diabetes mellitus (type I diabetes) has been obtained. The day of verification of diabetes mellitus was considered the zero-day of its development.Surgical procedure. On the day of verification, the skin surface of the right footpad was shaved and cleaned with a 70% ethanol wipe. On the skin of each rat’s right hind paw’s footpad, a full-thickness rectangular wound measuring 2 mm × 5 mm was created with a scalpel [14]. The scalpel and scissor wounds (Day 0) were of similar size and shape with minimal or no bleeding in all groups. Every day, the wounds were treated with the traditional method of treatment (5% alcohol solution of iodine and levomekol ointment), until the end of the experiment, also for the experimental group, in addition to the local traditional method of treatment, a new drug Reomannisol (JV LLC REKA-MED FARM, Republic of Uzbekistan) was used, which was administered intraperitoneally once a day for 5 days, in single doses of the therapeutic range for humans, taking into account differences in relative body surface area [1]. In all cases, the average dose of the studied range was 1 ml of Reomannisol per 100 g of the calculated equivalent mean therapeutic dose (EMTD).The development of the disease was assessed by the condition of the animals, lethality was recorded in groups, and recorded according to clinical symptoms (polyuria, polydipsia, polyphagia, weight loss, coat) and blood glucose levels. The wool of animals normally has a peculiar luster and is usually adjacent to the skin.The amount of water drunk by the rats was determined individually by measuring its volume with a measuring cylinder before and after the animals took water. To assess the daily values of diuresis, an individual urine collection was performed using urinals.Rats were taken out of the experiment by decapitation on days 1, 3, 7, 10, 14, blood was taken for laboratory examinations: biochemical analyzes (total protein, ALT, AST, glucose, creatinine, urea), products of lipid peroxidation (LPO) (malondialdehyde - MDA), indicators endogenous intoxication (MWM, SCE), morphological studies of tissues taken from the wound zone and the surrounding intact area were carried out. At each time, sampling for analysis was carried out in 10 animals of each group.Biochemical analyzes (theactivity of aspartate aminotransferase (AST), alanineaminotransferase (ALT), total protein, glucose, creatinine, urea) were measured on a biochemical analyzer photometer Mindray BA-88A (Mindray, China), using a set of chemical reagents manufactured by Human (Germany).Determination of malondialdehyde (MDA) using thiobarbituric acid. At high temperatures in an acidic medium, MDA reacts with 2-thiobarbituric acid, forming a colored trimethylene complex, with an absorption maximum at 532 nm on an SF-46 spectrophotometer (Russia). The molar coefficient was used - 1.56x105cm-1 x M-1. The MDA level was expressed in µmol/L [9].A biochemical method for determining endogenous intoxication, which consists in choosing the level of medium-weight molecules in the blood serum. Medium weight molecules (MWM) are an integral biochemical marker of endogenous intoxication (EI). In the blood plasma of all subjects, the level of MWM was assessed. For this, a screening method for determining molecules was used, this method includes taking blood, separating serum from blood cells by centrifugation, obtaining a protein-free sample by adding 0.5 ml of 10% trichloroacetic acid to 1 ml of blood serum, and centrifuging for 30 minutes at 3000 rpm in min. Next, 4.5 ml of distilled water is added to 0.5 ml of the supernatant and the optical density of the sample is determined on a spectrophotometer with a wave characteristic of 254 nm. The content of molecules of average mass is determined by the size of the optical sample, and the presence of endogenous intoxication is judged by their high degree. Measurement of the level of MWM and oligopeptides allows you to calculate the Integral index of intoxication. To calculate the optical density in the ultraviolet region of the spectrum, a UNICO 2800 spectrophotometer (USA) was used [2].Determination of the concentration of oligopeptides (OP). The method for determining the content of OP according to Lowry (1956) was used. To do this, 1 ml of a weakly acidic supernatant diluted 10 times with distilled water was taken, 2 ml of an alkali-copper reagent was added, and left for 10 min. at room temperature, then 0.2 ml of the Folin-CiocALTeu reagent was added, stirred, and after 30-40 minutes. the optical density was measured at 750 nm in a cell with a length of 5 mm on a UNICO 2800 spectrophotometer (USA) [20].The sorption capacity of erythrocytes (SCE) was determined by a method based on the idea of an erythrocyte as a universal adsorbent [10]. To do this, 1 ml of erythrocyte suspension was transferred into a test tube with 3 ml of 0.025% methylene blue in physiological saline. After a 10-minute incubation at room temperature, the sample was centrifuged for 10 minutes. at 3,000 rpm the optical density of the supernatant and the initial solution of methylene blue was determined concerning physiological saline at a wavelength of 630 nm using a UNICO 2800 spectrophotometer (USA). The amount of absorbed dye was expressed as a percentage [10].Evaluation of the effectiveness of the drug was carried out based on, also, a visual examination of the animals and their wounds. The criteria for the effectiveness of the drug for the wound were: focusing on the severity and duration of inflammatory manifestations in the wound area (edema, hyperemia, wound exudate), the state of the wound bottom; the appearance of granulation tissue; reduction in the area of the wound defect; the appearance of marginal epithelialization; acceleration of wound healing,The L.N. Popov test was used to determine the area of the wound. A sterile cellophane plate is placed on the wound and the contour of the wound is applied to it. The drawing is transferred to graph paper and the area of the wound is calculated.
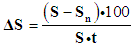 Where ΔS is the desired value in cm2;S is the area of the wound in the previous measurement in cm2;Sn - the size of the area of the wound at the moment in cm2;t is the number of days between measurements [5].The research methods described in the classical manuals on histomorphology were applied in the work [18]. Pieces from a diabetic foot, pancreas, and liver were fixed in Carnoy's solution (the composition of the fixative was glacial acetic acid, 10 parts; chloroform, 30 parts; ethyl alcohol, 60 parts). Fixing the pieces for 2-4 hours, then placing the pieces in 96% alcohol. Carrying out the wiring in the usual way and pouring in paraffin. Making histological sections with a thickness of 5-6 microns on a sledge microtome, deparaffinization on a thermostat, and staining with hematoxylin and eosin. It is the most common method of staining histological sections. Paraffin sections are deparaffinized in chloroform and washed in distilled water, then a hematoxylin solution is poured onto the sections for 3 minutes. Rinse in tap water for 10 minutes and the sections are stained with eosin from 0.2 to 3 minutes, depending on the thickness of the sections. They are dehydrated in alcohols of increasing concentration, starting from 70° to 96°, clarified in carbol-xylene, xylene, and placed in a balm. Result: cell nuclei are stained blue-violet, the cytoplasm is pink. The method of luminescent microscopic examination [19].
Where ΔS is the desired value in cm2;S is the area of the wound in the previous measurement in cm2;Sn - the size of the area of the wound at the moment in cm2;t is the number of days between measurements [5].The research methods described in the classical manuals on histomorphology were applied in the work [18]. Pieces from a diabetic foot, pancreas, and liver were fixed in Carnoy's solution (the composition of the fixative was glacial acetic acid, 10 parts; chloroform, 30 parts; ethyl alcohol, 60 parts). Fixing the pieces for 2-4 hours, then placing the pieces in 96% alcohol. Carrying out the wiring in the usual way and pouring in paraffin. Making histological sections with a thickness of 5-6 microns on a sledge microtome, deparaffinization on a thermostat, and staining with hematoxylin and eosin. It is the most common method of staining histological sections. Paraffin sections are deparaffinized in chloroform and washed in distilled water, then a hematoxylin solution is poured onto the sections for 3 minutes. Rinse in tap water for 10 minutes and the sections are stained with eosin from 0.2 to 3 minutes, depending on the thickness of the sections. They are dehydrated in alcohols of increasing concentration, starting from 70° to 96°, clarified in carbol-xylene, xylene, and placed in a balm. Result: cell nuclei are stained blue-violet, the cytoplasm is pink. The method of luminescent microscopic examination [19].4. Statistical Analysis
- The data obtained were statistically processed on a Pentium IV personal computer using the Microsoft Excel program. In addition, methods of traditional variational parametric and nonparametric statistics were used. To establish the reliability of the results obtained, the coefficient t - Student test was used. Differences were considered significant if the frequency for the studied trait did not exceed 5% (P <0.05). Statistical processing of digital data was carried out using the SPSS 16.0 and Statistica 6.0 for Windows application programs. Means and standard deviations, medians and interquartile intervals, as well as non-parametric methods (Mann-Whitney, Wilcoxon, Kruskal-Wallis tests,), were determined. Analysis of the probability of occurrence of the studied outcome in a certain period (survival) was performed according to the method of E. Kaplan - P. Meier. Cox stepwise regression analysis was used to identify several risk factors for survival. Analysis of the accuracy and practical value of prognostic factors and the validity of the models were measured by the method of concordant (c-statistic) statistics (estimation of the area under the ROC curve).
5. Results
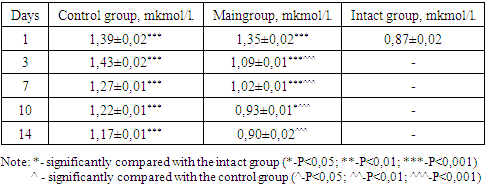
- The body bodyweight before the experiment varied from 220 to 250 g. Group 1 - intact animals (10 rats each), served as controls for groups 3 and 4. 2nd group –the creation of an experimental model of a diabetic foot, against the background of alloxan diabetes; To do this, 10 rats were injected intraperitoneally with 2% alloxan in an amount of 20 mg / 100 g. In this experimental group, in the 2nd experimental group, 8 rats died in the first 3 days as a result of hyperglycemic and hypoglycemic coma, which amounted to 80%. When examining the level of glucose in the blood with a glucometer of the remaining rats, it was 33.3 mmol/l and may have been higher, since the maximum range of the glucometer is 33.3 mmol/l. The remaining 2 rats sat in the corner, there were no reactions to external stimuli, they were sedentary when picked up. The animals did not touch the food. On the next day 4, the remaining rats died.The second series of the second group of the experimental model of diabetes mellitus was created based on alloxan at a dose of 15 mg per 100 g from 10 rats. In this series of experiments, in the first 3 days the lethality was 50% (5 rats). The glucose levels of the survivors (5 rats) ranged from 29.8 to 33.3 mmol/l. During the next 4 days, the remaining rats died.3rd series of the experiment of the 2nd group - administration of alloxan intraperitoneally at a dose of 12 mg per 100 g per 100 rats. During the next 72 hours, no lethal outcome was observed in rats, the range of blood glucose levels in rats varied between 15.5 - 17 mmol/L. In rats on the skin of the footpad pad of the right hind paw, a full-thickness rectangular wound measuring 2 mm × 5 mm was created with a scalpel. Rats were randomly divided into 2 groups, each group of 50 rats. So it was created 3 –a control group on 50 rats and 4 experimental groups n=50 rats. In both groups, until the end of the experiment (17 days), no death was recorded.Visual inspection. The first signs of diabetes were manifested in the form of a sharp increase in water consumption of 70-80 ml, polyphagia, polyuria, hyperglycemia. With alloxan-induced diabetes mellitus in animals during the experiment, lethargy, apathy, low activity, tarnishing and loss of coat, weight loss, clouding of the pupil and sclera, small-point erosion in the tails and limbs were noted. The wool of animals normally has a peculiar luster and is usually adjacent to the skin. In dynamic observation in rats of the experimental group, by the seventh day, the condition began to improve.In the study of indicators characterizing the state of various organs, for example, indicators of the state of liver cells and the degree of their damage are the activity of AST and ALT enzymes. In our studies, the activity of these enzymes in the blood of animals in both groups was significantly higher than in intact animals (ALT-34U/l ± 1.ASTAsT-33.4 U/l±1.1), Although, it should be noted that already after three injections of the drug rheomannisol, in the animals of the experimental group, the ALT and AST enzymes recorded low numbers (52.6±1.6; 48.4±1.4, respectively) compared with the control group (82.8± 1.4, 86.3±1.5). By the 7th day in the control group ALT-79 ± 0.ASTAsT-79±1.5, which is 1.8 times more than in the experimental group - ALT-44.7±0.94 AST AST-43, 3±1.0. In our study, in animals, the degree of elevation of the levels of enzymes alanine transferase and aspartate transferase indicates a pronounced violation of the cellular structure of the liver. Recorded activation of transaminases may indicate a violation of the integrity of hepatocyte membranes, leading to an increase in their permeability, and subsequently to the death of liver cells. A decrease in the activities of both enzymes (ALT, AST) followed the injections of rheomannisol in the experimental gr, out and on the 10th and 14th days, the numbers (ALT-37.5±0.62, AST-37.1±0.69; ALT-35.3± 0.54, AST-34.9 ± 1.04, respectively) indicate the normalization of the functional capacity of the liver, while in the control group, the activity of ALT and AST enzymes even on days 10, 14 (ALT-75.5 ± 1.1, AST-74.4±1.6; ALT-57.2±1.2, AST-53.4±1.3, respectively) 2 times higher than in the experimental one, and remain at a high level until the end of the experiment (Table 1).
|
|
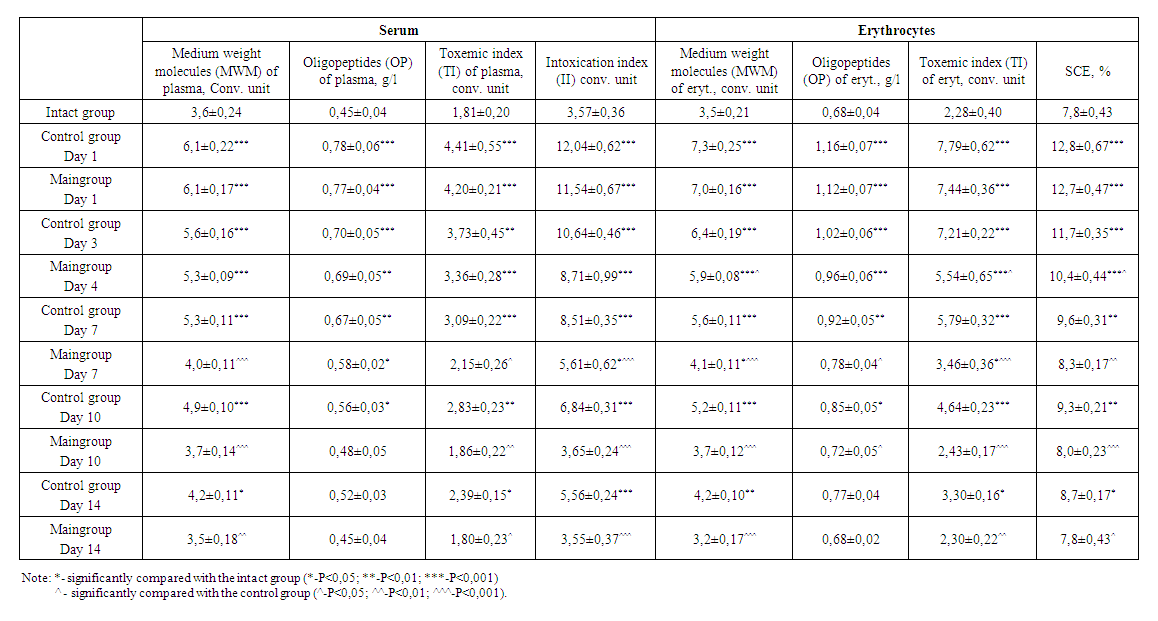 | Table 3. Dynamics of change in indicators of endogenous intoxication |
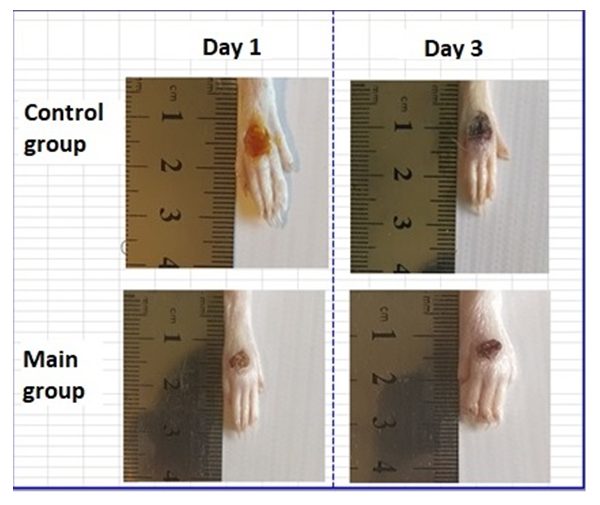 | Figure 1. Images of foot wounds on days 1, 3 after injury and comparison of the size (area) of wounds between control and experimental groups |
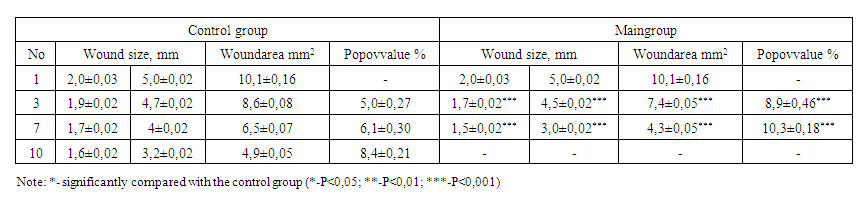 | Table 4. Comparative results of wound healing in animals with an experimental model of diabetic foot |
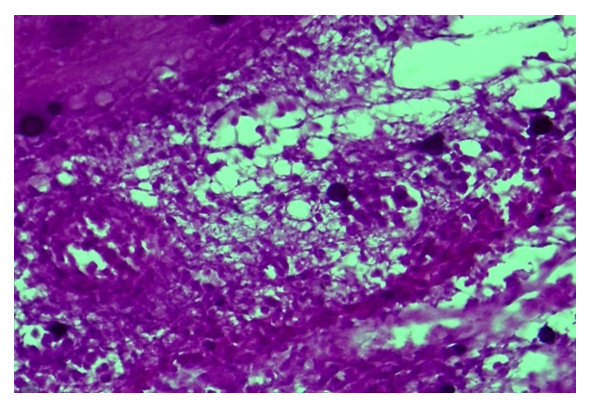 | Figure 2. Control group, Day 7. Diabetic wound. Preservation of necrotic mass and inflammatory infiltrate in the bottom of the diabetic foot. Staining: hematoxylin-eosin. Magnification: 10x40 |
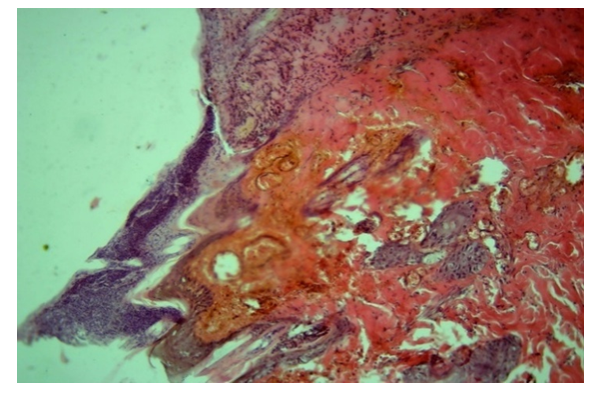 | Figure 3. Main group, Day 7. Rejection of necrotic tissue on the surface of a diabetic wound. Staining: hematoxylin-eosin. Magnification: 10x40 |
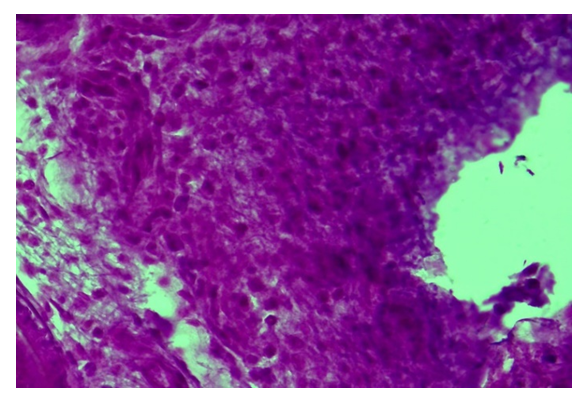 | Figure 4. Control group, Day 10. Diabetic wound. Formation of a dense inflammatory infiltrates around the wound of a diabetic foot. Staining: hematoxylin-eosin. Magnification: 10x40 |
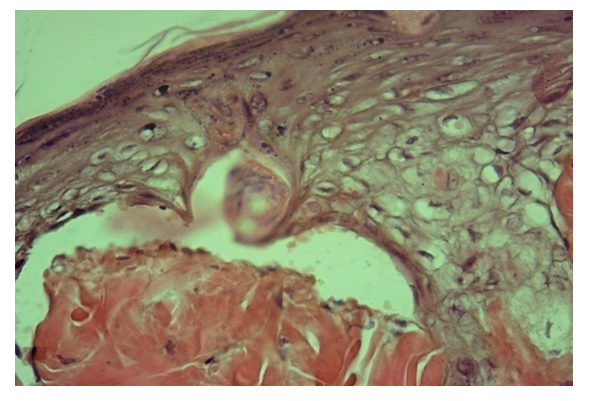 | Figure 5. Main group, day 10. Compaction of the surface layers of the epidermis, fibrosis of the connective tissue of the dermis. Staining: hematoxylin-eosin. Magnification: 10x40 |
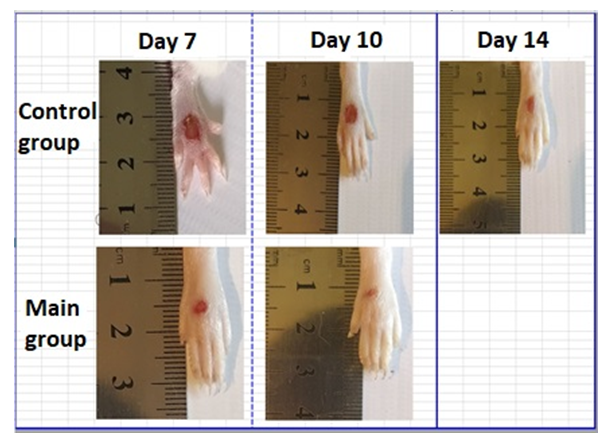 | Figure 6. Images of foot wounds on days 7, 10, 14 after injury and comparison of the size (area) of wounds between control and experimental groups |
6. Conclusions
- 1. The best option for creating an experimental model of a diabetic foot is the introduction of alloxan intraperitoneally in a single dose of 12 mg per 100 g, in which moderate diabetes develops.2. After using the drug reomannisol intraperitoneally at a dose of 1 ml / 100 g 1 time per day for 5 days, there was a sharp decline in EI numbers. On the 10th day, the EI values in the experimental group returned to normal, similar to those in the intact group. The drug reomannisol performs "biochemical rehabilitation", due to its inherent qualities: antioxidant, improves blood rheology, detoxification, and diuretic. In rats of the control group, the EI numbers remain at high levels until the end of the experiment.3. The results of biochemical studies demonstrate positive dynamics in experimental animals with a diabetic foot model when using the drug reomannisol. This was manifested by the fact that by the 10th day there was a decrease and normalization of the level of glucose in the peripheral blood, indicators of renal clearance (urea, creatinine), liver (ALT, AST, albumins).4. An open, full-thickness wound of the foot, in rats with DM, had low blood circulation, prolonged inflammation, and was characterized by a violation of the inflammatory and proliferative phases of the healing process, which is associated with hyperglycemia. Thus, this model of the open foot in rats provides a good approach for studying the process of wound healing in DM, and this model can be regarded as creating an analog of the human diabetic foot syndrome in an experimental model of alloxan-induced diabetes mellitus.5. The rate of healing of wound defects in rats with diabetic foot syndrome in the control group falls on the 14th day, since the terms of resorption and rejection of necrotic tissues in the wound are lengthened, damage to the vessels of the microvasculature (microangiopathy), edema is observed for a long time. The wounding process against the background of DM is characterized by the late formation of angiogenesis, slowing down and impaired maturation of granulation tissue, marginal epithelialization. In the experimental group, in rats, along with the local traditional method of wound treatment, the drug reomannisol was used intraperitoneally, as a result, wound healing was recorded on the 10th day from the moment the wound was applied to the foot of the rats. The use of local treatment and reomannisol can enhance angiogenesis in the early stages of the experiment and restore disturbed microcirculation (neoplasms of blood vessels), increase macrophage response, fibroblast proliferation, maturation and remodeling of granulation tissue and its epithelization, reduce the inflammatory reaction, which leads to more effective and early healing wound area.6. Comprehensive treatment (application of a local traditional method of treatment on the wound and the drug reomannisol) in an experimental model of the diabetic foot have positive effects on reparative processes and wound healing, due to the formation and enhancement of angiogenesis, as well as on the functional parameters of vital organs by reducing intoxication organism.
Ethical Approval
- Operations and all manipulations with animals were carried out using general anesthesia, in compliance with the principles of humanity outlined in the directives of the European Community (86/609/EEC) and the Declaration of Helsinki, by the "Rules for working with experimental animals". The ethical approval for the study was granted by Tashkent Medical Academy and the Committee of Ethical Approval for Researches under the Ministry of Health of the Republic of Uzbekistan.Conflict of interest. The authors declare that they have no competing interests.Funding. No funding sources to declare.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML