| [1] | Fraley, A. (2010). Citrus peel benefits, The American University of Washington, DC. June (2010). |
| [2] | Gonzalez-Molina, E., Dominguez-Peries, R., Moreno, C. and Garcia-Viguera, C. (2009). Natural bioactive compounds of citrus lemon for food and health. Elsevier, B.V., USA. |
| [3] | Manthey, A., and Grohmann, K. (2001). Phenols in citrus peel by-products. Concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. Journal of Agricultural and Food Chemistry, Vol. 49, No. 7, pp. 3268-3273. |
| [4] | Nogata, Y., Sakamoto, K., Shiratsuchi, H., Ishii, T., Yano, M. and Ohta, H. (2006). Flavonoid composition of fruit tissues of citrus species. Bioscience, Biotechnology and Biochemistry. Vol. 70, No. 1, pp. 178-192. |
| [5] | Cohen, E., Sharon, R., Volman, L., Hoenig, R., and Saguy, I. (2006). Characteristics of Israeli citrus peel and citrus juice. Ministry of Industry and Trade, Agriculture Research Organization, No. 869. State of Israel. |
| [6] | Thiel, S. (2011). The benefits of eating orange peel. http://www. livestrong.com/article/521033-the benefits-of eating orange peels/#ixzz21 fmbmknt. |
| [7] | Chedea, V.S., Kefalas, P., and Socacin, C. (2010). Patterns of carotenoid pigments extracted from two orange peel wastes (Valencia and Navel var.). Journal of Food Biochemistry. Vol. 34, No. 9, pp. 101-110. |
| [8] | Gross, J. (1997). Carotenoid pigments in citrus. In Citrus Science and Technology. Vol. 1 (S. Nagy, P.E. Shaw and M.K. Veldhus eds.). pp. 302-355. AVI Publishing, Westport. CT. |
| [9] | Rincon, A.M., Vasquez, A.M., and Padilla, F.C. (2005). Chemical composition and bioactive compounds of flour of orange (Citrus sinensis), tangerine (Citrus reticulate) and grapefruit (Citrus paradisl) peels cultivated in Venezuela. Arch. Latinoam Nutr., Vol. 55, No. 3, pp. 305-310. |
| [10] | Hui, S. (1999). Sweet oranges. The biogeography of citrus sinensis. http//Stephan cjb.net/resources/orange.html. |
| [11] | Marks, S. (2011). Nutrition information for dried orange peel. http:///www.livestrong.com/article/373022-nutrition-informtion-for-dried orange-peel/#ixzz 21 fm US MGD. |
| [12] | Marty, E. (2011). Do red and pink grapefruit reduce cholesterol. http://www.livestrong.com/article/392366-do-red-and-pink-grapefruit- reduce-cholesterol/#ixzzy 21 g 1T i2pz. |
| [13] | Huang, Y.S. and Ho, S.C. (2010). Polymethoxy flavones are responsible for the antiflammatory activity of citrus fruit peel. Food Chemistry, Vol. 119, No. 3, pp. 868-873. |
| [14] | Xu, C.J., Fraser, P.D., Wang, W.J. and Bramley, P.M. (2006). Differences in the carotenoid content of ordinary citrus and lycopene-accumulating mutants. Journal of Agricultural and Food Chemistry. Vol. 54, No. 12, pp. 5472-5481. |
| [15] | Starling, S. (2009). New citrus flavonoids debuted. William Reed Business Media. SAS. |
| [16] | Liu, Y., Ahmad, H., Luo, Y., Gardina, D.T., Guasekera, R.S., Mc Keehan, W.L. and Patil, B.S. (2001). Citrus pectin: Characterization and inhibitory effect on fiberblast growth factor-receptor interaction. J. Agric. Food Chem., 49 (6), 3051-3057. |
| [17] | Bobroff, L.B. (2002). Nutrition for health and fitness: Fiber in your diet. Sheet FCB 9130, a Series of the Department of Family, Youth and Community Sciences, Florida Cooperative Extensive Service. Institute of Food and Agricultural Sciences, University of Florida. |
| [18] | Nagappa, A.N., Thakurdesai, P.A., Rao, N.V. and Singh, J. (2003). Antidibetic activity of Terminalia catappa Linn fruits. Journal of Ethnopharmacology, Vol. 88, No. 1, pp. 45-50. |
| [19] | Murali, B., Upadhyaya, U.M. and Goyal, R.K. (2002). Effect of chronic treatment with Enicostemma littorale in non-insulin-dependent diabetic (NIDDM) rats. Journal of Ethnopharmacology. Vol. 18, No. 2, pp. 199-204. |
| [20] | Li, R.W. (2006). Citrus polymethoxylated flavones improve lipid and glucose homeostasis and adipocytokines in fructose – induced insulin resistant hamsters. Life Sciences, June, 2006. |
| [21] | Haldar, P.K., Kar, B., Bhattacharya, S., Bala, A., Kumar, S.R.B. (2010). Antidiabetic activity and modulation of antioxidant status by sansevieria roxburghiana rhizome in streptozotocin-induced diabetic rats. Diabetologia Croatica. Vol. 39, No. 4, pp. 115-123. |
| [22] | Oyelola, O.O., Moody, J.O., Odeniyi, M.A., and Fakeye, T.O. (2007). Hypolgycemic effect of Treculia Africana decne root bark in normal and alloxan-induced diabetic rats. African Journal of Traditional, Complementary and Alternative Medicines, Vol. 4, No. 4, pp. 387-391. |
| [23] | Panda, S.P., Haldar, P.K., Bera, S., Adhikary, S. and Kander, C.C. (2010). Antidiabetic and antioxidant activity of Swietenia mahagoni in streptozotocin-induced diabetic rats. Pharmaceutical Biology, Vol. 48, No. 9, pp. 947-979. |
| [24] | Horner, M. (2010). Grapefruit powder benefits, http://www.livestrong.com/article/243055-grapefruit-powder-benefits/#ixzz21g2heiYO. |
| [25] | Wolf, W. (2010). Health properties of orange peel. http://www.livestrong.com/article/346101.health-properties-of-orange-peel/#ixzz21998y Mr. |
| [26] | Kundusen, S., Haldar, P.K., Gupta, M., Mazumder, U.K., Saha, S., Bala, A., Bhattacharya, S. and Biswakanth, K. (2011). Evaluation of antihyperglycemic activity of citrus limetta fruit peel in streptozotocin-induced diabetic rats. International Scholarly Research Network, ISRN Endocrinology, Vol. 2011, Article ID 869273. p. 1-6. |
| [27] | Kameswrarao, B., Giri, R., Kesavalu, M.M., and Apparao, C. (1997). Herbal medicine, in the management of indigenous resources. J.B. Bajaj, Ed., Diabetes Mellitus in Developing Countries, pp. 375-377., Interprint, New Delhi, India. |
| [28] | Ali, M.M., and Abd El-Kader, M.A. (2004). The influence of naringin on the oxidative state of rats with streptozotocin-induced acute hyperglycaemia, zeitschrift fur Naturforschung. Section C, Vol. 59, No. 9-10, pp. 726-733. |
| [29] | Jung, J., Lee, M.K., Jeong, K.S. and Choi, M.S. (2004). The hypoglycaemic effects of hesprin and naringin are partly mediated by hepatic glucose regulating enzymes in C57 BL/KSJ-db/db mice. Journal of Nutrition. Vol. 134, No. 10, pp. 2499. |
| [30] | Amerman, D. (2011). Benefits of citrus peel extract. http://www.livestrong.com/article/490637-benefits-of-citrus-peel-extract/#ixzz21fx Nobow. |
| [31] | Michael, Li, St. (2010). The benefits of grapefruit powder supplement. http://www.livestrong.com/article/276582-the-benefits-of-grapefruit-powder-supplement/#ixzz21g4Mj1NN. |
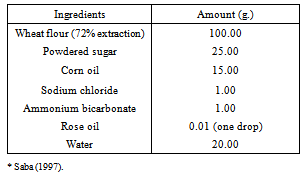
| [32] | Saba, N.H. (1997). Culinary is Science and Art. P. 685. Dar El-Maaref-Cairo (In Arabic). |
| [33] | Vetsala, C.N., and Haridas Rao, P. (1991). Studies on invert sugar for use in biscuits. J. Food Sci. & Techn. 28: 149-152. |
| [34] | Manohar, R.S. and Rao, P.H. (1997). Effect of mixing period and additives on the rheological characteristics of dough and quality of biscuit. J. of Cereal Sci., 25: 197-206. |
| [35] | El-Sayed, H.H.M. (2001). Biological studies on effect of Portulaca deracea L. on lowering lipids and blood sugar in experimental animals. Ph.D. Thesis, Faculty of Home Economics, Minufiya University, Minufiya. |
| [36] | Ilwy, Y.M.E. (2003). The effect of some kinds of sea food (fish) on blood lipid profile in rats. Ph.D. Thesis, Faculty of Specific Education, Ain Shams University. |
| [37] | Anon. (1980). Second Report of the American Institute of Nutrition. J. Nutrition: 110: No. 8, 1726. |
| [38] | Tietz, N.W. (1995). Clinical Guide to Laboratory tests. 3rd ed. Philadelphia. WB. Saunders, 268-273. |
| [39] | SAS Institute (1990). SAS users Guide in Statistics, Cary, NC, SAS Institute, USA. |
| [40] | Perez, Y.Y., Jimenez-Ferrer, E., Alonso, D., Botello-Amaro, A. and Zamilpa, A. (2010). Citrus limetta leaves extract antagonizes the hypertensive effect of angiotension II, Journal of Ethnopharmacology, Vol. 128, No. 3, pp. 611-614. |
| [41] | Harborn, J.B. (2001). Chemical Dictionary of Economic Plants. July 2001. USA. |
| [42] | Fujioka, K. (2006). The effects of grapefruit on weight and insulin resistance: relationship to the metabolic syndrome. J. Med. Food, 9 (1): 49-54. |

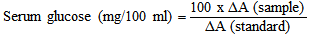 where 100 = The concentration of the standard solution and ΔA = The absorbance.
where 100 = The concentration of the standard solution and ΔA = The absorbance.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML