-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2020; 10(2): 26-32
doi:10.5923/j.chemistry.20201002.02
Received: Sep. 6, 2020; Accepted: Sep. 28, 2020; Published: Oct. 15, 2020

DABCO Catalyzed One-Pot Synthesis of Ester Derivatives of Tris (2-Aminoethyl)amine
Md. Wahab Khan, Laila Arifun Nahar
Department of Chemistry, Bangladesh University of Engineering & Technology (BUET), Dhaka
Correspondence to: Md. Wahab Khan, Department of Chemistry, Bangladesh University of Engineering & Technology (BUET), Dhaka.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

 Tris (2-aminoethyl) amine (Tren) is a commercially available tripodal amine. Tren has been widely used as a ligand in the preparation of metal complexes. Tris (2-aminoethyl) amine (Tren)-based esters have been synthesized in a single-pot-reaction of tren with six equivalent each of alkyl acrylates, 3-trimethoxysilyl propyl methacrylate and 2-hydroxyethylmethacrylate in presence of 10 mol% DABCO in mild conditions. The higher yield of the product was obtained in case of alkyl acrylates. The reaction was accomplished at room temperature and in a short reaction time. All synthesized compounds were characterized by IR, UV, 1H NMR, 13C NMR, GC-mass and elemental analysis.
Tris (2-aminoethyl) amine (Tren) is a commercially available tripodal amine. Tren has been widely used as a ligand in the preparation of metal complexes. Tris (2-aminoethyl) amine (Tren)-based esters have been synthesized in a single-pot-reaction of tren with six equivalent each of alkyl acrylates, 3-trimethoxysilyl propyl methacrylate and 2-hydroxyethylmethacrylate in presence of 10 mol% DABCO in mild conditions. The higher yield of the product was obtained in case of alkyl acrylates. The reaction was accomplished at room temperature and in a short reaction time. All synthesized compounds were characterized by IR, UV, 1H NMR, 13C NMR, GC-mass and elemental analysis.
Keywords: Tris (2-aminoethyl) amine, Alkylacrylates, 1, 4-Diaza bicyclo [2. 2. 2] octane (DABCO), Ester
Cite this paper: Md. Wahab Khan, Laila Arifun Nahar, DABCO Catalyzed One-Pot Synthesis of Ester Derivatives of Tris (2-Aminoethyl)amine, American Journal of Chemistry, Vol. 10 No. 2, 2020, pp. 26-32. doi: 10.5923/j.chemistry.20201002.02.
Article Outline
1. Introduction
- Tris (2-aminoethyl) amine (Tren) [N, N-Bis (2-aminoethyl)-1, 2-ethane diamine] is a commercially available tripodal amine. Tren has been widely used as a ligand in the preparation of metal complexes [1-3]. Sterically hindered triamidoamine ligands with a C3 symmetrical tren-based backbone Tren have been found to coordinate to a variety of transition metals stabilizing unusual complexes thereby allowing exceptional reactivities [4,5]. Ligands of the type [(RNCH2CH2)3N3- (R=H [6], methyl [7], benzyl [8]) have been shown to form complexes with transition metals or main group elements [9,10]. Over the past several years triamidoamine ligands based on tris (2-aminoethyl) amine (Tren) have been explored extensively to coordinate a variety of transition metals forming important complexes which showed versatile activities [11-15]. Greco and Schrock reported the synthesis of triamidoamine ligands of the type (ArylNHCH2CH2)3N and Molybdenum and Tungsten complexes that contain an [(ArylNCH2CH2)3N]3- ligand [16]. The copper (II) complexes [Cu(Bz3tren)H2O](ClO4)2 and [Cu(Bz3tren)Cl]Cl were synthesized and structurally characterized [17] by M. Schatz et al. Mösch-Zanetti et al. reported the synthesis of a C6F5 substituted unsymmetrical triamidoamine ligand along with the preparation of two rhenium (V) oxo compounds [18]. Recently, Guillaume Barre et al. have prepared the hexa substituted tetramine ligand [19] by refluxing tris (2-aminoethyl) amine, 1-iodooctadecane and potassium carbonate in acetonitrile for 2 days and they have reported its catalytic effects for living radical polymerization of methylmethacrylate. It has been reported that tris (2-aminoethyl) amine based tetradentate chelating ligand forms stable complexes with transition metals which is used as a carbon dioxide absorbent [20]. The synthesis of cationic lipids, tris (2-aminoethyl) amine-based α-branched fatty acid amides has been reported resulting a series of lipids with specific variations in the lipophilic as well as the hydrophilic part of the lipids [21]. Tripodal ligand based on tris (2-aminoethyl) amine was prepared that served as potential Fe (III) binding chelator and to detect Fe for further modification to develop a better potent fluorescence sensor [22]. In this paper, a novel approach to synthesize ester derivatives of tris (2-aminoethyl) amine is described.
2. Experimental
2.1. General Remarks
- Melting points were determined in open capillary tube on Gallenkamp (England) melting point apparatus and uncorrected. IR spectra were recorded on a Shimadzu FTIR spectrophotometer and UV spectra were recorded in dry MeOH with Shimadzu visible spectrophotometer. 1H NMR and 13C NMR spectra were recorded on a Bruker DPX-400 spectrophotometer (400 MHZ) using tetramethylsilane as internal reference and solvent CDCl3. Elemental analyses (C, H, N) were carried out on a Perkin-Elmer 240 C Analyser. Analytical thin-layer chromatography (TLC) was performed on precoated silica gel 60F-254 (E. Merck), and the spots were visualized with UV light. Column chromatography was performed on silica gel (60-120 mesh). DABCO (1, 4-diazabicyclo [2. 2. 2] octane), tris (2-aminoethyl) amine, acrylates, 3-(trimethoxysilyl) propylmethacrylate, 2-hydroxyethyl methacrylate and other reagents were purchased from E. Merck (Germany) and Fluka (Switzerland).
2.2. General Procedure for the Synthesis of Compound (7-11)
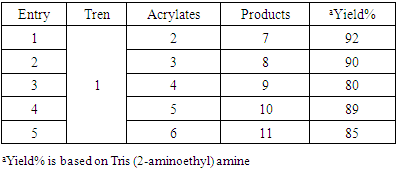
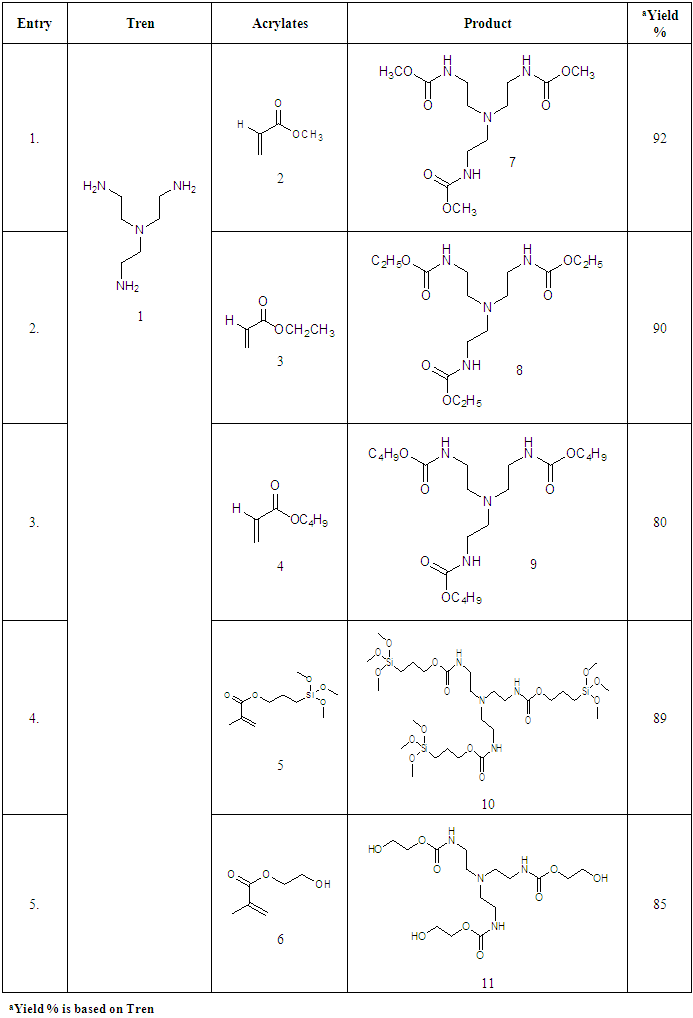
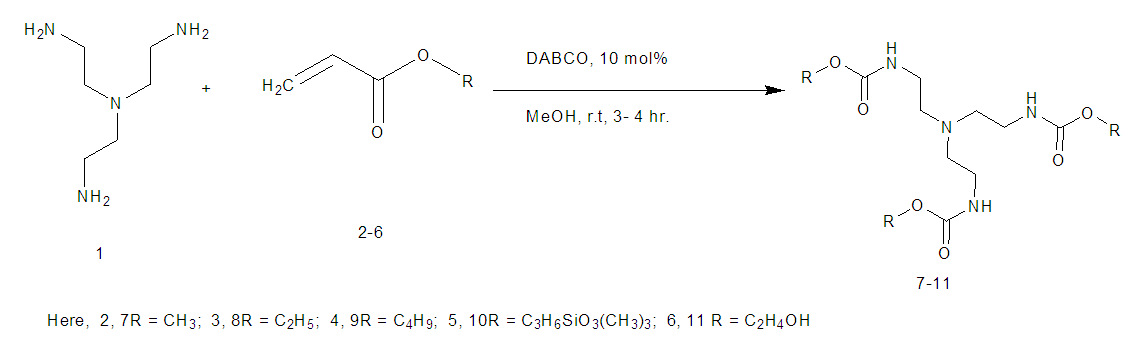
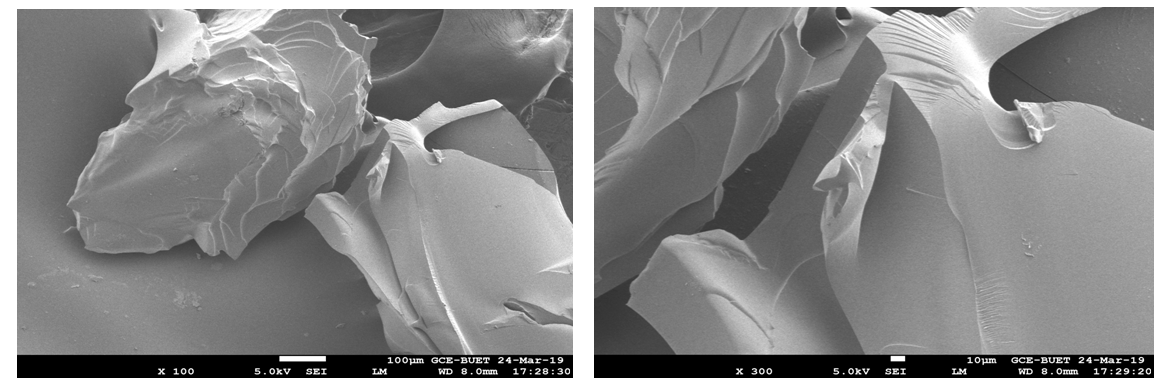
- Compounds 7-11 were prepared from the reaction of tris (2-aminoethyl) amine with different acrylates in presence of DABCO at room temperature. To a stirred solution of tris (2-aminoethyl) amine (0.5 g, 0.0034 mol) and DABCO (0.038 g, 10 mol %) in methanol (5 mL) at r. t. and methyl acrylate (1.90 g, 0.02 mol) was added. Then the mixture was stirred for 3-4 hours. The progress of the reaction was monitored by TLC. Then the solvent of the reaction mixture was removed by distillation and extracted by CHCl3 solution (3 x 50 ML), the organic layer was washed by distilled water (3 x 50 ML) and dried over anhydrous Na2SO4. The crude product obtained after evaporation of the solvent was purified by chromatography on a column of silica gel (60-120 mesh) with ethyl acetate in n-hexane to yield yellowish liquid pure compound 7.Compound 7: Yellowish liquid, Yield: 92%, IR (KBr): νmax = 3448.84 (N-H), 2845.10-2956.01 (C-H), 1736.96 (C=O), 1438.94 (CH2), 1330.93-1364.68 (C-N), 1202.66 (-O-), 700-1000 cm-1. UV (MeOH): λmax = 335.20, 280.80, 231.80 nm; 1H NMR (400 MHz, CDCl3): δ (ppm) 3.68 (s, 3H, CH3), 2.55 (s, 1H, NH), 2.80 (t, 2H, J = 6.8 Hz, CON-CH2), 2.48 (t, 2H, J = 6.8, 7.2 Hz, NCH2). 13C NMR (100 MHz, CDCl3): δ (ppm) 172.87, 51.57, 49.65, 32.44. HRMS (CI) Calcd for C12H24N4O6 [M+H]+ 320.34, found [M+H]+ 320.49. Anal. Calcd. (%) for C12H24N4O6: C 44.99, H 7.55, N 17.49; Found (%) C 45.12, H 7.38, N 17.68.Compound 8: The product was obtained from tris (2-aminoethyl) amine (0.5 g, 0.0034 mol), ethyl acrylate (2.21 g, 0.02 mol) and DABCO (0.038 g, 10 mol %). Yellowish liquid, Yield: 90%, IR (KBr) 34.50 (N-H), 2826.77-2890.05 (C-H), 1740 (C=O), 1469.81 (CH2), 1372.40 (C-N), 1190.30 (-O-) cm-1; UV 340.30, 282.20, 232.00, 230.80 nm; 1H NMR (400 MHz, CDCl3): δ (ppm) 4.11 (q, 2H, J = 7.2, 6.8 Hz, OCH2), 2.78 (t, 2H, J = 7.2 Hz, CON-CH2), 2.44 (t, 2H, J = 7.2 Hz, NCH2), 2.55(s, 1H, NH), 1.25 (t, 3H, J = 7.2 Hz, CH3); 13C NMR (100 MHz, CDCl3): δ (ppm) 172.49, 60.44, 49.72, 32.71, 14.22. HRMS (CI) calcd for C15H30N4O6 [M+H]+ 362.42, found [M+H]+ 362.29. Anal. Calcd. (%) for C15H30N4O6: C 49.71, H 8.34, N 15.46; Found (%) C 49.74, H 8.43, N, 15.62.Compound 9: The product was obtained from tris (2-aminoethyl) amine (0.5 g, 0.0034 mol), butyl acrylate (2.82 g, 0.02 mol) and DABCO (0.038 g, 10 mol %). Yellowish liquid, Yield: 80%, IR 34.88.13 (N-H), 2840.10-2880.83 (C-H), 1736.00 (CO), 1462.09 (CH2), 1280-1320 (C-N), 1180 (-O-) cm-1; UV (MeOH): λmax = 279.60, 231.60 nm; 1H NMR (400 MHz, CDCl3): δ (ppm) 4.04 (t, 2H, J = 6.8 Hz, OCH2), 2.76 (t, 2H, J = 6.8 Hz, CON-CH2), 2.52 (s, 1H, NH), 2.42 (t, 2H, J = 7. 2, 6.8 Hz, NCH2), 1.60 (quint, 2H, J = 6.8 Hz, aliphatic H-3), 1.36 (quint, 2H, J = 7.2, 7.6 Hz, aliphatic H-2), 0.930 (t, 3H, J = 7.2, 7.6 Hz, CH3); 13C NMR (100 MHz, CDCl3): δ (ppm) 172.54, 64.25, 49.72, 32.67, 30.67, 19.12, 13.68. HRMS (CI) calcd for C21H42N4O6 [M+H]+ 446.58, found [M+H]+ 446.51. Anal. Calcd. (%) for C21H42N4O6: C 56.48, H 9.48, N 12.55; Found (%) C 56.65, H 9.30, N 12.72.Compound 10-11 were also prepared through the above reaction procedure. After usual workup the solid compounds were obtained without any separation technique. It is mentioned here that the compound 10-11 were not soluble in any organic solvent.Compound 10: The product was obtained from tris (2-aminoethyl) amine (0.5 g, 0.0034 mol), 3-(trimethoxysilyl) propyl methacrylate (5.47 g, 0.02 mol) and DABCO (0.038 g, 10 mol %). Yield: 89%, Colourless solid, mp 187-188 °C: IR 3467.16 (N-H), 2933.83 (C-H), 1726.35 (CO), 1460.16 (CH2), 1280.05 (-O-), 940.33 (Si-O) cm-1. The SEM and EDX of this compound were carried out. Compound 11: The product was obtained from tris (2-aminoethyl) amine (0.5 g, 0.0034 mol), 2-hydroxyethyl methacrylate (2.87 g, 0.02 mol) and DABCO (0.038 g, 10 mol %). Yellowish solid, Yield: 85%, mp 145-146°C: IR 3537.57 (N-H), 3417.01 (OH), 2853.78-2954.22 (C-H), 1725.38 (CO), 1462.09 (CH2), 1274.03 (C-N), 1151.54 (-O-) cm-1. The SEM and EDX of this compound were taken.
3. Results and Discussion
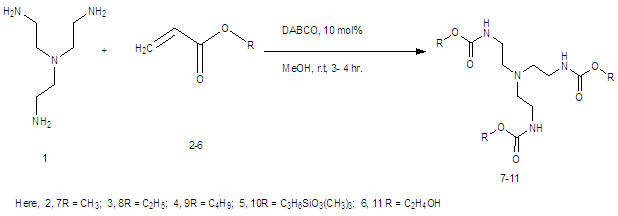
- It is reported here that the coupling between tris (2-amino ethyl) amine with acrylates were accomplished in presence of DABCO in good yield% (Scheme 1, Table 1). A divergent synthesis of the polyamidoamine type compound by using two monomers is developed. A straightforward methodology to highly substituted alkyltren ester compounds is demonstrated. A single pot base catalyzed synthetic protocol has been employed to assemble alkylacrylates, 3-(trimethoxysilyl) propyl methacrylate, 2-hydroxy- ethylmethacrylate, with Tren to obtain Tren base ester macro molecules. The reaction of a central Tren unit bearing three primary amine moieties with six equiv. each of alkyl acrylates provides access to a series of functionalyzed tripodal esters (Scheme 1). Tris (2-aminoethyl) amine is taken as the core molecule and alkyl acrylate is considered as coupling monomer. The additions of three alkylacrylate molecules to tris (2-aminoethyl) amine in solvent MeOH was occurred rapidly in presence of DABCO. The nucleuophilic addition involves by the electron pair on the nitrogen to the electropositive carbonyl carbon with release of alkene group.
|
 | Scheme 1 |
|
 | Figure 1. SEM for the compound 10 by applying from 5.0 kv 7.2 mm x 50 (SEM) to 5.0 kv 7.2mm x 20,000 (SEM) |
 | Figure 2. EDX spectra of the compound 10 |
 | Figure 3. SEM for the compound 11 by applying from 5.0 kv 8 mm x 100 (SEM) to 5.0 kv 8 mm x 1,000 (SEM) |
 | Figure 4. EDX spectra of the compound 11 |
4. Mechanism
- It can be perceived that the reactions proceed according to Scheme-2 although the detailed mechanism of the reaction is yet to be clarified. It was observed that the presence of DABCO was very essential for the success of this C-N bond forming reactions. Tris (2-aminoethyl) amine is taken as the core molecule and alkyl acrylate is considered as coupling monomer. The additions of three alkylacrylate molecules to Tris (2-aminoethyl) amine in solvent MeOH was occurred rapidly in presence of DABCO. The nucleuophilic addition involves by the electron pair on the nitrogen to the electropositive carbonyl carbon with release of alkene group.
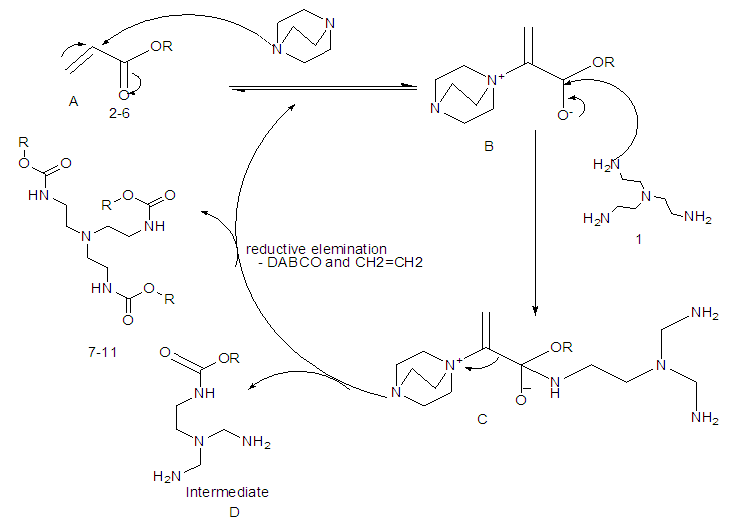 | Scheme 2 |
5. Conclusions
- A convenient method for the synthesis of macro molecule of ester derivatives from the reaction of Tris (2-aminoethyl) amine with acrylates using 10 mol% 1, 4-Diaza bicyclo [2. 2. 2] octane (DABCO) in MeOH has been developed under mild conditions at room temperature. The synthesized compounds would be important ligands and potent intermediate for the synthesis of medicinal compounds. The most important features of these methods were that, the readily available inexpensive materials were used under relatively mild conditions and got relatively higher yield. No toxic and hazardous compounds were produced by this procedure. Therefore, this methodology could be utilized to synthesize the biologically important dendrimer type compounds in mild conditions.
ACKNOWLEDGEMENTS
- We gratefully acknowledge the financial support of Bangladesh University of Engineering and Technology (BUET), Dhaka-1000, Bangladesh.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML