-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2020; 10(2): 33-37
doi:10.5923/j.chemistry.20201002.03
Received: Sep. 16, 2020; Accepted: Oct. 2, 2020; Published: Oct. 15, 2020

Schiff Base Complex of Cu (II) with Antibacterial and Electrochemical Study
Arjun C. Bhowmick 1, Majharul I. Moim 1, Miththira Balasingam 2
1Department of Chemistry, Mawlana Bhashani Science and Technology University, Santosh, Tangail, Bangladesh
2Analytical Research Division, Syncrude Canada Limited, Calgary, Alberta, Canada
Correspondence to: Arjun C. Bhowmick , Department of Chemistry, Mawlana Bhashani Science and Technology University, Santosh, Tangail, Bangladesh.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The complex N, N'-Disalicylideneethylenediaminecopper (2) has been prepared and the structure of the complex is confirmed from the IR, 1H NMR, and Elemental analysis. In solid state the complex packs as a dimer with square pyramidal geometry. A reversible cyclic voltammogram (CV) is found from an electrochemical study that involves one electron redox process. The antibacterial activity of the complex is investigated against E. Coli and Shigella Dysenteriae gram negative bacteria where streptomycin antibiotic is used as a standard, and the complex has shown good inhibition towards the growth of these gram-negative bacteria.
Keywords: Bacteria, Conductance, Complex, Ligand, Antibiotic
Cite this paper: Arjun C. Bhowmick , Majharul I. Moim , Miththira Balasingam , Schiff Base Complex of Cu (II) with Antibacterial and Electrochemical Study, American Journal of Chemistry, Vol. 10 No. 2, 2020, pp. 33-37. doi: 10.5923/j.chemistry.20201002.03.
Article Outline
1. Introduction
- The synthesis and study of Schiff base metal complexes [1] were started in the year 1984 since they have some potential applications as a catalyst [2-5], binding DNA [6], cleaving DNA [7-9], anticancer [10-11], antitumor [12-14] and antioxidants [15-20]. It was also noted that these class of compounds show some interesting magnetic [21] and optical [22-23] properties. Additionally, Schiff base metal complexes are being studied for oxygen reduction catalyst that is important in fuel cell [24-25] technology and in biological system [26]. Recently, a group has concentrated for using metal Schiff base complexes to track sub terranean fluid flow [27]. Considering the importance of their versatile applicability Schiff base metal complexes are still being studied by various research groups all over the world [28-33].In this article, it is demonstrating the synthesis and characterization of Cu(salen) Schiff base [34] complex (2). This will be a possible application as a catalyst [35-38] in the future. This complex may also be possible to apply as an antimicrobial agent [39]. Previously, the complex was reported by a few research groups [40-42]. It exists as a dimeric form in solid state, and in solution it may exist as a smaller proportion of dimeric form or may be in the equilibrium [43-44] between dimer and monomer. However, it has been noted that from the birth of the Schiff base chemistry it has synthesized a large family of different types of Schiff base metal complexes and some of them have been published without having any potential application. In this article, it is aimed to synthesize the Cu(salen) complex (2) again to investigate its antibacterial and electrochemical properties. It is believed that this research will be helpful for the future development of antibacterial drugs against various types of gram-negative bacteria. Additionally, electrochemical study will be helpful for the future development of electrocatalyst.
2. Experimental
2.1. Material and Measurements
- All chemicals were purchased from commercially available sources and used without further purification. The conductance was measured by using Elico-conductometer. The IR spectrum was recorded by Perkin-Elmer spectrophotometer in KBr pellets. The UV-Visible spectrum was recorded in CHCl3 on Beckman DU-64 spectrophotometer with quartz cells of 1 cm path length. 1H NMR spectrum was recorded in CDCl3 by Bruker Advance 400 MHz instrument. Melting point was determined from Fisher apparatus (up to 350°C).
2.2. Synthesis of the of Schiff Base
- 30 ml of acidic (2 ml glacial acetic acid) ethanolic solution of salicylaldehyde (8.1412g, 66.66 mmol) was stirred for 10 min [26]. To the solution, ethylinediamine (2g, 33.28 mmol) was added drop wise, and finally, yellow color N, N'-bis(salicylidene)ethlenediamine was collected by filtering (Scheme 1). The whole solution was stirred for 4h at RT. The resulting crude product was recrystallized in CHCl3 at room temperature. Spectral data: Anal. Cal. For C16H16N2O2: C, 71.63; H, 6.01; N, 10.44. Found: C, 70.69; H, 6.19; N, 9.91. IR (υ CO, KBr): 3455 (OH, b), 3052 (ArC-H, w), 3014 (N=C-H, w), 2937 (CH2, w), 2860 (CH2, w), 1636 (C=N, vs), 1578 (ArC=C, s), 1198 (C-C, s), 1283 (C-O, m), 1148 (C-N, vs). 1H NMR (CDCl3): δ 13.2 (2H, s), δ 7.3-6.9 (8H, m), 8.4 (2H, s), 3.9 (4H, S).
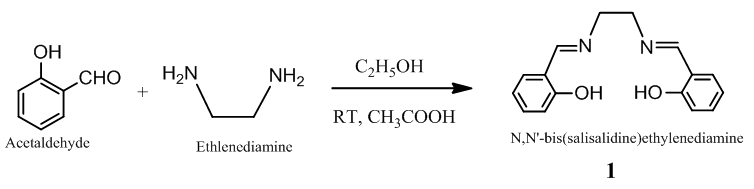 | Scheme 1. Synthesis of N, N’-bis(salisalidine)ethylene- diamine Schiff base |
2.3. Synthesis of Metal Complex
- 0.4658 g (1.87 mmol) of CuSO4. 5H2O was dissolved in 20 mL DMSO (dimethyl sulfoxide) and stirred for 30 min, and in another pot 0.500 g (1.87 mmol) of N, N′-Ethylenebis (salicylimine) (1) was also dissolved in 10 ml of DMSO. The Schiff base 1 was added to the metal salt solution and the resulting solution was refluxed for 4h. Finally, black crystalline material was separated and recrystallized in CHCl3 (0.63g, 77.46%) (scheme 2). Spectral data: Anal. Cal. For C32H28N4O4Cu2: C, 58.25; H, 4.28; N, 8.49. Found: C, 56.72; H, 4.44; N, 8.04. IR (υ CO, KBr):3444 (H2O-OH, b), 3052 (ArC-H, w), 3018 (N=CH, s) 2929(CH2, w), 2853 (CH2, w), 1647 (C=N, vs), 1528 (ArC=C,s), 1191 (C-C, s), 1233 (C-O, m), 1137 (C-N, vs), 561 (Cu-N), 465 (Cu-O, m). 1H NMR (CDCl3): δ 7.0-7.4 (8H, m), δ 8.1 (2H, s) δ 3.4 (4H, s), δ 1.6 (2H-H2O, s). Melting Pont: >300°C. Molecular conductance (μs/cm): 8.6 (DMSO), 9.6 (MeOH), 0 (CHCl3). UV-Visible (λmax, CHCl3): 244, 275, 366, 570.
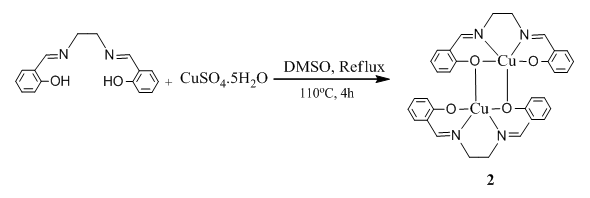 | Scheme 2. Synthesis of N, N'-Disalicylideneethylene- diaminecopper |
3. Result and Discussion
- The synthesis of the Schiff base metal complex (2) are involved in two steps. The first step is the synthesis of the Schiff base ligand (1) (section 2.2, scheme 1). Next, this ligand is used for complexing with metal (section 2.3, scheme 2). The complexation reaction was done in solvent DMSO to achieve higher temperature. The IR and 1H NMR data of the ligand is shown in section 2.2.The structure was investigated by infrared spectroscopy (IR) and the aromatic C-H gave stretching at 3052 cm-1, imine C=N was found at 1647 cm-1, aromatic C=C gave at 1528 cm-1. The aliphatic ethylene group C-H symmetric and asymmetric stretching were found 2853 cm-1 and 2929 cm-1, respectively. The details of other respective IR stretching values are given in the section 2.3. The 1H NMR was recorded in CDCl3, but due to poor solubility and the paramagnetic nature of the Cu (II) metal, the spectrum is not looking good. The aromatic protons gave multiplets at δ 7.0-7.4 (8H) and the two imine CH=N proton gave singlet at δ 8.1. The four ethylene protons gave singlet at δ 3.4 (4H, s). The melting point of the complex was determined and found above 300°C. The UV-Visible spectrum was recorded in CHCl3 and two absorption peaks were detected at 275 and 366. The molecular conductivity was measured, and the conductivity was found very small (section 2.3). So, it is possible to conclude that the complex 2 acts as a poor electrolyte.
4. Electrochemical Study
- The CV of the complex 2 was recorded from 0 to -2 V (Figure 1) with scan rate 100 mV/s under Ar atmosphere and it went through a reversible redox process that involves one electron. This indicates that CuII goes to CuI after receiving an electron during negative scan, and later giving back that electron, it turns into the +2-oxidation state. The peak current
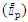 was found 11 µA. But, when it is first oxidized, the process involves two electrons (Figure 2). May be this is due to the CuII to CuIII state [35] and the residual ligand moiety may also oxidize. 10 mL of 0.1M [nBu4N]BF4 in dimethyl sulfoxide (DMSO) was used as electrolyte during studying electrochemistry. The glassy carbon was used as the working electrode and quasi Ag/AgCl was used as the reference electrode and counter electrode used Pt wire. The cyclic voltammogram indicates that the complex in the solution acts as monomer since the electrochemical process involves only one electron [35]. The CV of the complex 2 was also studied in MeCN and found similar result as in DMSO.
was found 11 µA. But, when it is first oxidized, the process involves two electrons (Figure 2). May be this is due to the CuII to CuIII state [35] and the residual ligand moiety may also oxidize. 10 mL of 0.1M [nBu4N]BF4 in dimethyl sulfoxide (DMSO) was used as electrolyte during studying electrochemistry. The glassy carbon was used as the working electrode and quasi Ag/AgCl was used as the reference electrode and counter electrode used Pt wire. The cyclic voltammogram indicates that the complex in the solution acts as monomer since the electrochemical process involves only one electron [35]. The CV of the complex 2 was also studied in MeCN and found similar result as in DMSO.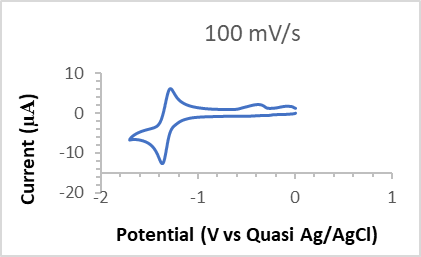 | Figure 1. Cyclic voltammogram (CV) (Red to Ox) of the 0.1 mM Cu(salen) complex (2), the glassy carbon working electrode, Quasi Ag/AgCl reference electrode |
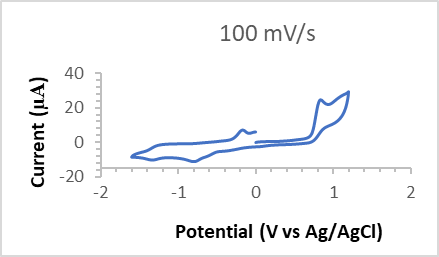 | Figure 2. Cyclic voltammogram (CV) (Ox to Red) of the 0.1 mM Cu(salen) complex (2), the glassy carbon working electrode, Quasi Ag/AgCl reference electrode |
5. Antibacterial Study
5.1. Antibacterial Activity in Muller Hinton Agar Media
- 11.4 g of Muller Hinton agar was dissolved in 300 ml of distilled water, and after shaking was put into autoclave for ½ h at 121°C. The resulting solution was poured into eight plates (disk) and the plates rested for 10 min. 10μl E. coli gram negative bacteria were applied to all disks uniformly and at the center of a plate standard antibiotic streptomycin was placed. In a test tube, 1mg of metal complex dissolved in 1ml CHCl3, and 10μl of the sample was dropped in the bacterial disks. It happens that CHCl3 inhibits the bacterial growth, and in a separate disk, 10μl CHCl3 was dropped to know the inhibition by CHCl3. All procedures were repeated for Shigella Sonnei, gram negative bacteria.
5.2. Antibacterial Study of the Cu(salen) (2)
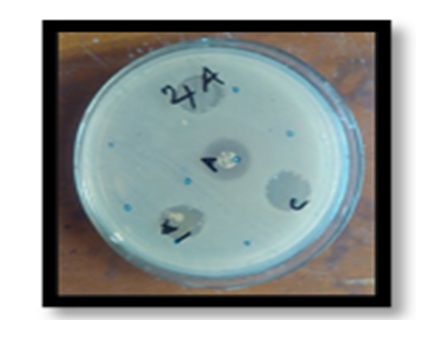
- The complex was tested against two-gram negative bacteria E. Coli and Shigella Sonnei. The complex inhibited the growth of E. Coli bacteria and found the length of inhibition around 40 mm. Alternately, the complex showed negative result for Shigella Sonnei. The E. Coli and the Shigella Sonnei were grown in Muller Hinton Agar (MHA) media. The detail of the process is described in the supporting information (Figure 3 and Figure 4) and streptomycin was used as the standard.
 | Figure 4. Antibacterial study of the Cu(salen) complex: the bacteria is E. Coli in Muller Hinton Agar; 10 M, 12M is Cu(salen) complex, C is CHCl3 |
6. Conclusions
- The synthesis of the Cu(salen) complex (2) has been successful and has found good results from electrochemical and antibacterial studies. Antibacterial study suggests the complex is effective to inhibit the growth of E. Coli. The electrochemistry study suggests the complex will be good for one electron redox process. In the future, more research will be focused to give potential applications of some other Schiff base metal complexes.
ACKNOWLEDGEMENTS
- We are thankful to the Department of Chemistry, Mawlana Bhashani Science and Technology University, Santosh, Tangail-1902, Bangladesh for funding this Project. We are also grateful to Professor Miles Koppang, Department of Chemistry, University of South Dakota for his guidance of doing electrochemical study. It is also a big pleasure of us of having Mr. Abu Zaffar Shibly, Assistant Professor, Department of Biotechnology and Genetic Engineering, Mawlana Bhashani Science and Technology University, Bangladesh, for helping the antibacterial studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML