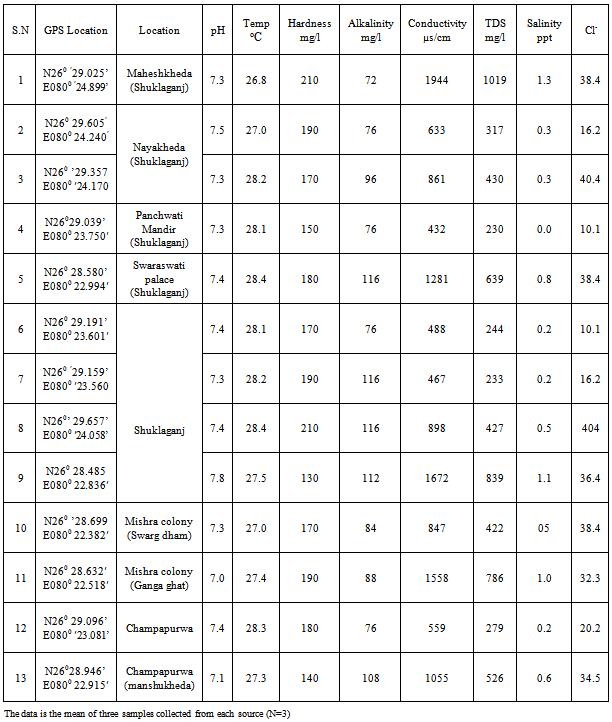
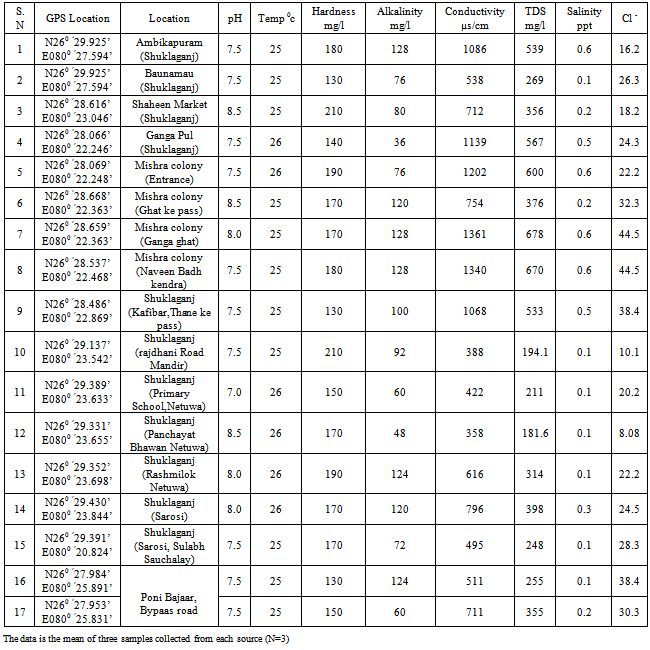
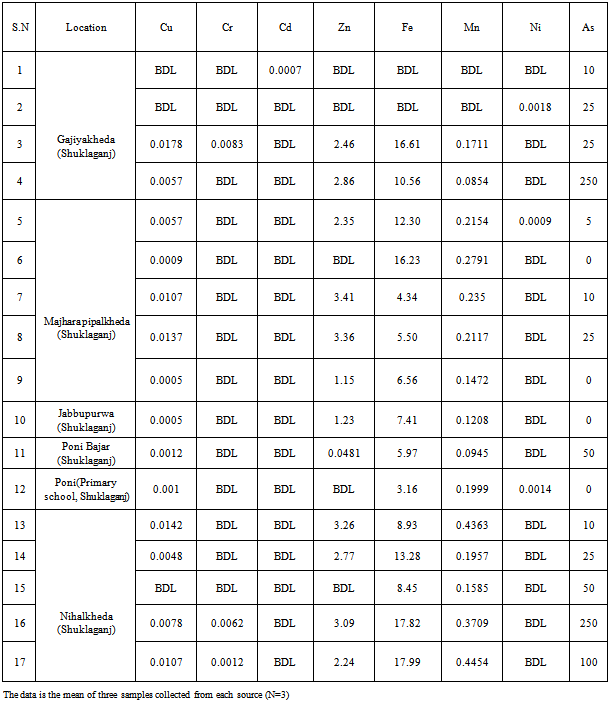
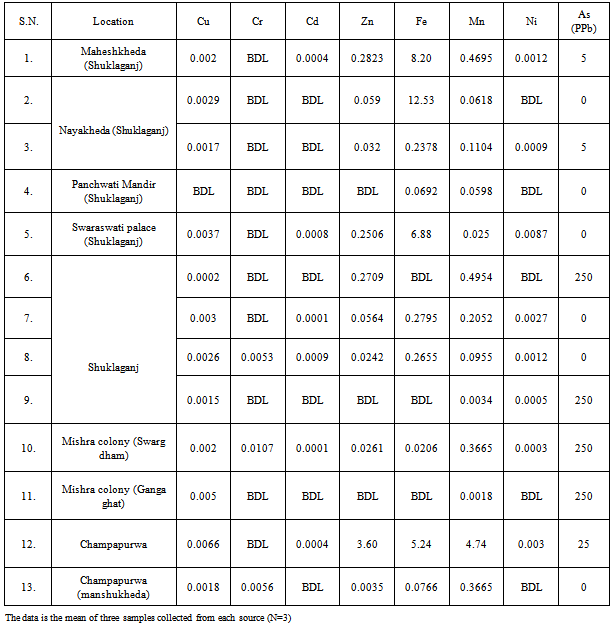
| [1] | USEPA, National primary drinking water regulations, arsenic and clarifications to compliance and new source contaminants monitoring final rule. Fed. Regis. 66 6976, 2001. |
| [2] | World Health Organising. Guidelines for drinking water Quality. In Recommendations, vol 1, WHO, Geneva, 1993. |
| [3] | P.L.Smedley, D.G. Kinniburgh, A review of the source, behavior and distribution of arsenic in natural waters. Appl. Geochem. 17, 517-568, 2002. |
| [4] | Leonard A.. Arsenic. In “Metals and Their Compounds in the Environments: Occurrence, Analysis, and Biological Relevance (E. Merian, Ed.), 2nd edn, pp. 751–773, 1991.Weinheim, VCH. |
| [5] | A. H.Smith, M. Goycolea, R. Haque, M.L.Biggs, Marked increase in bladder and lung cancer mortality in a region of northern Chile due to arsenic in drinking water.Am. J. Epidemiol. 147, 660–669, 1998. |
| [6] | USEPA, United States Environmental Protection Agency, Integrated Risk InformationSystem on Arsenic. Office Health Environ Assess, Cincinnati, OH.Adriano, D. C., Page, A. L., Elseewi, A. A., Chang, A. C., and Straughan, I. (1980). Utilization and disposal of fly ash and other residues in terrestrial ecosystems: A review. J. Environ.Qual. 9, 333–344, 1993. |
| [7] | Adriano D..C..,“Trace Elements in Terrestrial EnvironmentsBiogeochemistry,Bioavailability and Risks of Metals”, 2nd edn, Springer, New York, 2001. |
| [8] | D.Chakraborti, M.M. Rahman, K. Paul, U. K., Chowdhury, M.K. Sengupta, D. Lodh, C.R.Chanda, K.C., Saha, S.C. Mukherjee, Arsenic calamity in the Indian subcontinent: What lessons have been learned? Talanta 58, 3–22, 2002. |
| [9] | A. Chatterjee, D.Das, B.K. Mandal, T.R. Chowdhury, G. Samanta, D.Chakraborti, Arsenic in ground-water in 6 districts of West-Bengal, India - the biggest arsenic calamity in the world. 1. Arsenic species in drinking-water and urine of the affected people. Analyst 120, 643–650 ,1995. |
| [10] | A.H.Smith, O.E. Lingas, M. Rahman, Contamination of drinking water by arsenic in Bangladesh: A public health emergency. Bull. World Health Organ. 78, 1093–1103, 2000. |
| [11] | G.Wang, Arsenic poisoning from drinking water in Xinjiang. Chin. J. Prevent. Med. 18, 105–107, 1984. |
| [12] | M.Berg, H.C. Tran, T.C. Nguyen, H.V. Pham, R.Schertenleib, W. Giger, Arsenic contamination of groundwater and drinking water in Vietnam: A human health threat. Environ. Sci. Technol. 35, 2621–2626, 2001. |
| [13] | F.J.Lu, Blackfoot disease: Arsenic or humic acid? Lancet 336, 115–119,1990. |
| [14] | A.H.Smith, M.Goycolea, R. Haque, M.L. Biggs, Marked increase in bladder and lung cancer mortality in a region of northern Chile due to arsenic in drinking water. Am. J. Epidemiol. 147, 660–669, 1998. |
| [15] | R.C.Hopenhayn, M.L.Biggs, D.A.Kalman, L.E.Moore, A.H.Smith, Arsenic methylation patterns before and after changing from high to lower concentrations of arsenic in drinking water. Environ. Health Perspect. 104, 1200–1207, 1996. |
| [16] | L.M.Del Razo, M.A. Rellano, M.E. Cebrian, The oxidation states of arsenic in well-water from a chronic arsenicism area of northern Mexico. Environ. Pollut. 64, 143–153, 1990. |
| [17] | M.A. Khan, Y.S.Ho, Arsenic in drinking water : A review on toxicological effects, mechanism of accumulation and remediation. As. J.Chem.23,1889-1901, 2011. |
| [18] | K.D. Huysmans, W.T.Jr Frankenberger, Evolution of trimethylarsine by a Penicillium sp. Isolated from agricultural evaporation pond water. Sci. Total Environ. 105, 13–28, 1991. |
| [19] | M.Styblo, L.M.Del Razo, L.Vega, D.R.Germolec, E.L.Le Cluyse, G.A.Hamilton,W.Reed, C.Wang, W.R. Cullen and D.J. Thomas, Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells.Arch. Toxicol. 74, 289, 2000. |
| [20] | F.W. Pointus, K.G.Brown, C.J.Chen, Adsorption of arsenic onto hydrous ferric oxide: effects of adsorbate/adsorbent ratios and co-occurring solutes.J.Am Water Work Assoc. 86, 52, 1994. |
| [21] | National Research Council Uptake : National Academy Press : Washington DC 2001. http://www.nap.edu/books/030907 6293/html. |
| [22] | APHA, Standard method for the examination of water and wastewater 21st edition. American Public Health Asso. Washington DC, 2005. |
| [23] | N.E.Korte, Q.Fernando, A review of arsenic (III) in groundwater. Crit. Rev.Environ. Control. 21, 1–39, 1991. |
| [24] | W.R.Penrose, Arsenic in the marine and aquatic environments: Analysis, occurrence and significance. CRC Crit. Rev. Environ. Control 4, 465–482, 1974. |
| [25] | D.G.Smith, Heavy metals in the New Zealand aquatic environment: A review. National Water and Soil Conservation Authority, Wellington, New Zealand. Water and Soil Miscellaneous Publication No. 100, 1986. |
| [26] | A.L.Foster, G.E. Brown, G. A Parks, T.N. Tingle, D.E. Voigt, S.L. Brantley, XAFS determination of As(V) associated with Fe(III) oxyhydroxides in weathered mine tailings and contaminated soil from California, USA. J. De Physique IV 7, 815–816, 1997. |
| [27] | W.H.Clement, S.D.Faust, The release of arsenic from contaminate sediments and muds. J. Environ. Sci. Health 1, 87–91, 1981. |
| [28] | F.N.Robertson, Arsenic in groundwater under oxidizing conditions, southwest United States. Environ. Geochem. Health 11, 171–185, 1989. |
| [29] | P.L.Smedley, H.B.Nicolli, D.M.J. Macdonald, A.J. Barros, J.O.Tullio, Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl. Geochem. 17, 259–284, 2002. |

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML