-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2012; 2(5): 139-143
doi: 10.5923/j.als.20120205.03
Antimicrobial activity of extracts obtained from Urera baccifera (L.) Gaudich
Sideney Becker Onofre , Patricia Fernanda Herkert
Paranaense University , UNIPAR , Unit of Francisco Beltrão , PR. Av. Julio Assis Cavalheiro, 2000, Bairro Industrial , 85601-000 , Francisco Beltrão , Paraná , Brazil
Correspondence to: Sideney Becker Onofre , Paranaense University , UNIPAR , Unit of Francisco Beltrão , PR. Av. Julio Assis Cavalheiro, 2000, Bairro Industrial , 85601-000 , Francisco Beltrão , Paraná , Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The purpose of this work was to assess the antimicrobial activity of extracts from Urera baccifera. Aqueous, ethanol and methanol extracts made from the leaves, bark and roots of U. baccifera were tested, at different concentrations using the disk diffusion method, against the bacteria Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 27853). Inoculated plates were incubated at 35º C ± 1 C for 24 hours and the inhibition halos were assessed and interpreted. The methanol extracts from the leaves (ML) and roots (MR) had greatest antimicrobial activity against the three bacteria tested. The MICs of the ML and MR extracts against E. coli were 6.25 and 0.19 mg/L, respectively, and against P. aeruginosa and S. aureus they were 3.12 and 0.19 mg/L (for both species). The results show that the methanol extracts of the leaves and roots of U. baccifera are antimicrobially active against E. coli, P. aeruginosa and S. aureus.
Keywords: Antimicrobial, Natural Products, Medicinal Plants, Secondary Metabolites
Cite this paper: Sideney Becker Onofre , Patricia Fernanda Herkert , "Antimicrobial activity of extracts obtained from Urera baccifera (L.) Gaudich", Advances in Life Sciences, Vol. 2 No. 5, 2012, pp. 139-143. doi: 10.5923/j.als.20120205.03.
Article Outline
1. Introduction
- Since antiquity medicinal plants have been used in the treatment of several illnesses that afflict humans. This type of medicine corresponds to any plant-derived matter that is administered to a living being and presents a pharmacological property for the treatment of a pathological condition[1].Within the pharmaceutical industry, medicinal plants represent a primary source of substances that are used to create new medicines. Plants are considered natural biochemical laboratories that synthesize several active principles[2], such as phenols, quinones, flavonoids, tannins, coumarins, terpenes, alkaloids and lectins, which have antimicrobial activity in vitro and can be extracted by organic solvents or water[3].The use of plants in medicine is currently of great interest due to how difficult it is to treat some diseases, the resistance of microorganisms[4] and the increased cost of synthetic medications[5] (Fuck et al., 2005). Although there are several types of antimicrobials in clinical use, many are ineffective against some types of microorganisms because of the ability of these organisms to quickly develop a resistance to the medicine[6].For these reasons, research is focused on finding new active principles that can be used in the production of new medications. Considering the high biodiversity in Brazil, the popular know-how about the properties of medicinal plants and the unknown chemical characteristics of most species, the scientific assessment of the therapeutic value of plants with still-unknown properties is becoming increasingly promising[5].Urera baccifera (Urticaceae)[7] (Figure 1) is a perennial, erect shrub, ranging in height from 1.5 to 2.5 m, with simple, alternate leaves, which have stinging hairs that cause skin burns. The flowers are small, unisexual, and grow in axillary inflorescences. The fruits are small, achene- or drupe-shaped[8], spongy, hydrated and rich in carbohydrates and proteins[9]. This species is popularly known as nettle and can be found along forest edges in Tropical America, where there is a humid, shady environment[10].Among many components, the leaves of U. baccifera contain proteins (23% of the total), calcium (5%), potassium (3.1%), magnesium (0.54%), phosphorus (0.27%), sulfur (0.27%), iron (0.0209 mg.kg-1), sodium (0.0108 mg.kg-1), manganese (0.0072 mg.kg-1), boron (0.0053 mg.kg-1), zinc (0.0039 mg.kg-1) and copper (0.0008 mg.kg-1)[11].Martins et al.[12] tested the activity of extracts of the aerial parts of U. baccifera against the herpes simplex virus (HSV) and observed that these extracts only had activity against HSV-1, and not HSV-2. They also reported that butanol extracts showed high antiviral and viricide activity (85.9%), and that extracts of ethyl acetate inhibited the cellular receptors by 90% and viral penetration by 85.9%, but did not show viricide activity. In addition, the ethanol extracts in their study showed antiviral activity because they inhibited the virus from penetrating the cells (94.4%).
 | Figure 1. Urera baccifera (L.) Gaudich. ex Wedd. – Urticaceae |
2. Materials and Methods
2.1. Collection Site
- The plant material was collected in the countryside in the town of Dionísio Cerqueira, Santa Catarina, Brazil (26°24’17.19’’S, l53°38’49.27’’; elevation 511m). The specimens were identified and stored in the collection at the Botanical Laboratory at Paranaense University, Campus of Francisco Beltrão, PR. When the collections arrived at the laboratory, material was selected by separating the leaves, trunk and roots, which were dried in a stove for seven days at 60º C ±4. The material was then ground and stored in paper bags in a dry environment. All of the collections were authorized by IBAMA under the permit number13.234-2 (1 August, 2006).
2.2. Obtainment of the Ethanol and Methanol Extracts
- The extracts were obtained by macerating 30 g of each dried part of the plant and then immersing the material in 300 mL of 99.8% methanol or 96% ethanol, for 24 hours, after the solution was quickly boiled. The material was then filtered and concentrated in an exhaustion chamber at room temperature until about 20% of the initial volume was left. The finished extracts were kept in amber flasks at room temperature until use[18].
2.3. Obtainment of the Aqueous Extract
- To obtain the aqueous extracts, 50 g of each part of the plant were separately added to 500 mL of sterile distilled water. The material was kept at rest at room temperature, for 24 hours, after the solution was quickly boiled. Next, the extracts were filtered and concentrated in an exhaustion chamber at room temperature until the concentrated solution was 20% of the initial volume. The extracts were kept in amber flasks at room temperature until use[19].
2.4. Preparation of the Samples
- Flasks were numbered from 1 to 10 according to the different concentrations prepared. dimethylsulphoxide (DMSO) was employed as a solvent for the ethanol and methanol extracts, and water was employed for the aqueous extracts. The concentrations prepared were 100, 50, 25, 12.5, 6.25, 3.12, 1.56, 0.78, 0.39 and 0.19 mg/L. Next, discs of filter paper, 6 mm in diameter, were soaked with the extracts and placed on the surface of the culture medium.
2.5. Preparation of the Inoculate
- For the antimicrobial assessments, strains from the American Type Culture Collection (ATCC) that belong to the Microbiology Laboratory at UNIPAR were used: Staphylococcus aureus (ATCC 25923), a microorganism that dwells in skin, mucosa and upper respiratory tract and can cause cutaneous and even systemic infections; Escherichia coli (ATCC 25922), a member of the intestinal microbiota, is involved in enteritis, urinary infections and nosocomial bacteremias; and Pseudomonas aeruginosa (ATCC 27853), an important opportunistic bacterium, is involved in hospital and urinary infections and in sepsis[20]. The microbial cultures were standardized to tube 0.5 of the McFarland scale.
2.6. Test of Sensitivity
- The method used was that of Kirby Bauer (disk diffusion), which involves the inoculation of a standard solution of a specific microorganism on an agar surface. Paper disks, previously saturated with the samples whose antimicrobial activity is to be investigated, are placed on the agar. The substances impregnated on the paper disks diffuse through the culture medium and, if the sample has inhibitory activity against the tested microorganism, an inhibition halo forms around the disk. After the incubation period, defined specifically for the microorganism, the zones of inhibition are measured[21-22].The antibiotics Amoxicillin 10 mg and Chloramphenicol 30 mg (Newprov® ) were used as positive controls. The negative controls were Dimethyl Sulphoxide, Methanol 99.8% and Ethanol 96%.
2.7. Essay of Disk Diffusion
- The suspensions of microorganisms were inoculated on plates containing Mueller-Hinton (MH) agar. The disks containing the extracts were then transferred to the media containing each inoculate. The plates were incubated at 35º C ± 1 for 24 hours. After this period, they were inspected for the presence of inhibition halos and these, when present, were measured (in mm).
2.8. Broth Dilution Method
- This method was used to determine the MIC (Minimum Inhibitory Concentration) of bioactive samples was carried out using serial dilutions in the ratio 1:2, using 2 ml of BHI broth. Two groups were controls, positive and negative, respectively, formed by the culture medium (BHI) plus 2 μL of microbial suspensions, and this culture medium without the addition of the inoculum[23-25].The samples were solubilized in a solution of dimethylsulfoxide (DMSO) 25%. Because the intensity of color of the material analyzed, which alter the color in the medium, making the visual reading of the results was added 0.2 mL of triphenyl tetrazolium chloride (TTC) 2%.The Minimal Inhibitory Concentration (MIC) was considered as the lowest concentration of the extract capable of inhibiting microbial development.
3. Results and Discussion
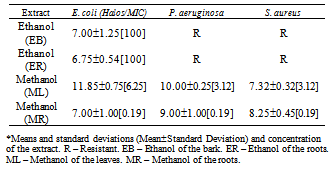
- From the nine extracts assessed in this work, only four showed antimicrobial activity. These were the ethanol extract from the bark (EB), the ethanol extract from the roots (ER), the methanol extract from the leaves (ML) and the methanol extract from the roots (MR). These data are shown in Table and Figure 2.An analysis of this data revealed that most of the antimicrobial activity of the four extracts was against Escherichia coli, because all four extracts inhibited its growth. The MIC was 100 mg/L for the EB, exhibiting a 7.00±1.25 mm inhibition halo. This behavior was also observed with the ER at the concentration of 100%, which had a 6.75±0.54 mm inhibition halo. The methanol extracts of the leaves and roots showed higher inhibitions, with halos of 11.85±0.75 mm[MIC 6.25] and 7.00±1.00 mm[MIC 0.19], respectively. The EB and ER extracts did not exhibit antimicrobial activity against P. aeruginosa and S. aureus. On the other hand, the ML and MR extracts inhibited their growth, with MICs of 3.12 and 0.19 mg/L (for both). The inhibition halos for P. aeruginosa were 10.00±0.25 mm and 9.00±1.00 mm, and for S. aureus they were 7.32±0.32 mm and 8.25±0.45 mm, respectively.The MR reached 38 mm against S. aureus, 37 mm against P. aeruginosa and 29 mm against E. coli at 100 mg/L concentration. The inhibitory activity against the first two bacteria was higher than that of the control antibiotics.
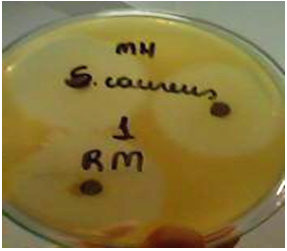 | Figure 2. Activity on the root extract against S. aureus - (ATCC 25923) |
4. Conclusions
- Based on data collected in this study, it can concluded that, among the extracts assessed, the ethanol extracts from the bark and the root of Urera baccifera (L.) Gaudich only had antimicrobial activity against Escherichia coli (ATCC 25922) and that the methanol extracts obtained from the leaves and roots had antimicrobial activity against Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853) and Staphylococcus aureus (ATCC 25923).
Conflict of Interest
References
| [1] | G. M. Rocha, M. E. M. Rocha, Uso popular de plantas medicinais. Saúde e Ambiente em Revista, vol.1, p.76-85, 2006. |
| [2] | C.L. Moura, Avaliação da Atividade Antimicrobiana dos Extratos Brutos das Espécies Vegetais Miconia rubiginosa e Pfaffia glomerata em Microrganismos da Cavidade Bucal, 2006, M.S. Thesis, Universidade de Franca, Franca, São Paulo, Brasil. |
| [3] | M. M. Cowan, Plant Products as Antimicrobial Agents. Clinical Microbiology Reviews, v.12, p.564-582, 1999. |
| [4] | T. M. Souza-Moreira, H; R. N. Sangalo, R. C. L. R. Pietro, O Brasil no contexto do controle de qualidade de plantas medicinais. Rev. Bras. Farmacogn, vol.20, p.435-440, 2010. |
| [5] | S.B. Fuck et al. Plantas medicinais utilizadas na medicina popular por moradores da área urbana de Bandeirantes, PR, Brasil. Ciências Agrárias, vol.26, p.291-296, 2005. |
| [6] | A. Bertucci,et al. Initial antimicrobial activity studies of plants of the riverside forests of the southern Uruguay Rev. Bras. Farmacogn, vol.19, p.20-25, 2009. |
| [7] | Life-Catologue of life. Disponível em: |
| [8] | A. B. Joly, Botânica Introdução à Taxonomia Vegetal. 13th Edn. Companhia Editora Nacional, São Paulo, 2002, p.239. |
| [9] | H. P. Dutra, A. L. V. Freitas, P. S. Oliveira, Dual and attraction in the neotropical shrub Urera baccifera (Urticaceae): the role of ant visitation to pearl bodies and fruits in herbivore deterrence and leaf longevity. Funtional Ecology, vol.20, p.252-260, 2006. |
| [10] | H. Lorenzi, Plantas Daninhas do Brasil. 3th Edn. Instituto Plantarum. São Paulo, 2000, p: 593. |
| [11] | V. F. KINUPP, I. B. I. BARROS, Teores de proteína e minerais de espécies nativas, potenciais hortaliças e frutas. Ciênc. Tecnol. Aliment, vol.28, p.846-857, 2008. |
| [12] | F. O. Martins et al. In vitro inhibitory efect of Urera baccifera (L) Gaudich. extract against Herpes Simplex. Afr. J. Pharm. Pharmacol, vol.3, p.581-584, 2009. |
| [13] | V. Kock, Estudo etnobotânico das plantas medicinais na cultura italobrasileira no Rio Grande do Sul: Um modelo para o cultivo comercial na agricultura familiar, M.S. Thesis, 2000. 128p. Universidade Federal do Rio Grande do Sul, Porto Alegre, RS. Brasil. |
| [14] | F. Q. Oliveira, L. A. Gonçalves, Conhecimento sobre Plantas Medicinais e Fitoterápicos e Potencial de Toxicidade por Usuários de Belo Horizonte, Minas Gerais. Rev. Eletrônica de Farmácia, vol.3, p.36-41, 2006. |
| [15] | G. Kavalali et al. Hypoglycemic activity of Urtica pilulifera in streptozotocin-diabetic rats. J. Ethnopharmacol, vol.84, p.241-245, 2002. |
| [16] | L. Testai, et al. Cardiovascular effects of Urtica dioica L. (Urticaceae) roots extracts: in vitro and in vivo pharmacological studies. J. Ethnopharmacol, vol.81, p.105-109, 2002. |
| [17] | M. R. E. Uncini, L. Zaccaro, P; E. Tomei, Antiviral activity in vitro of Urtica dioica L., Parietaria diffusa M. K. and Sambucus nigra L. J. Ethnopharmacol, vol.98, p.323-327, 2005. |
| [18] | P. I. Ushimaru, et al. Antibacterial activity of medicinal plant extracts. Brazilian Journal of Microbiology, vol.38, p.717-719, 2007. |
| [19] | C. T. Lubian, et al. Atividade antifúngica do extrato aquoso de Arctium minus (Hill) Bernh. (Asteraceae) sobre espécies orais de Candida. Rev. Bras. Pl. Med, vol.12, p.157-162, 2010. |
| [20] | C. C. Taveira, Ação antimicrobiana de extratos de plantas do Cerrado e isolamento de substância ativa de Kielmeyera coriácea, 2007. 112p. M.S. Thesis, Universidade de Brasília, Brasília, DF. Brasil. |
| [21] | H. D. Isenberg, Clinical Microbiology Procedures Handbook. American Society for Microbiology. Washington, D.C., 2001, p.657. |
| [22] | E. W. Koneman, et al. Provas de Sensibilidade a Agentes Antimicrobianos. In: Diagnóstico Microbiológico, Texto e Atlas Colorido. 5th Edn. Medsi. Rio de Janeiro, p. 828-829, 2001. |
| [23] | C.V. Nakamura, et al. Antibacterial activity of Ocimum gratissimum L. essential oil. Memórias do Instituto Oswaldo Cruz, vol. 94, no. 5, pp. 675-678, 1999. |
| [24] | CLSI - Clinical and Laboratory Standard Institute - 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, Wayene, Pa. |
| [25] | J. H. Jorgensen, J. D. Turnidge, Susceptibility test methods: Dilution and disk diffusion methods. In: MURRAY PR (ed) Manual of Clinical Microbiology. 8th ed. New York: ASM International. 2003. pp:1108-1127. |
| [26] | P. A. Meléndres, V. A. Capriles, Antibacterial properties of tropical plants from Puerto Rico. Phytomedicine, vol.13, p.272-276, 2006. |
| [27] | P. R. C. Castro, R. A. Kluge. L. E. P. Peres, Manual de Fisiologia Vegetal, Teoria e Prática. Agronômica Ceres. Piracicaba, p.338-339, 2005. |
| [28] | L. Taiz, E. Zeiger, Fisiologia Vegetal. 3th Edn. Artmed. Porto Alegre, p.309-327, 2004. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML