-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2014; 4(2): 54-62
doi:10.5923/j.ajps.20140402.05
Formation and Properties of Poly (vinyl butyral-co-vinyl alcohol-co-vinyl acetate) / Polystyrene Composites Reinforced with Graphene Oxide-Nanodiamond
Ayesha Kausar
Nanosciences and Catalysis Division, National Centre For Physics, Quaid-i-Azam University Campus, 44000, Islamabad, Pakistan
Correspondence to: Ayesha Kausar, Nanosciences and Catalysis Division, National Centre For Physics, Quaid-i-Azam University Campus, 44000, Islamabad, Pakistan.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
A facile and cost-effective method was used to prepare poly(vinyl butyral-co-vinyl alcohol-co-vinyl acetate) (PVBVA)/polystyrene (PS)/graphene oxide (GO) and graphene oxide-nanodiamond (GO-ND)-based nanocomposites. Formation of GO, nanodiamond functionalization of GO and nanocomposite structure were confirmed using FTIR. SEM imaging of GO, GO-ND and PVBVA/PS/GO-ND nanocomposites revealed significant results. The results revealed that nanodiamond functional GO platelets were fully incorporated into matrix. Mechanical studies depicted higher tensile strength (31.6-35.1 MPa) for PVBVA/PS/GO-ND nanocomposites compared with PVBVA/PS/GO without nanodiamonds (22.1-28.3 MPa). Thermogravimetric analysis showed higher 10% degradation temperature for nano-bifiller reinforced PVBVA/PS/ GO-ND 0.1-5 as 532-554ºC. Limiting oxygen index and UL 94 results depicted that PVBVA/PS/GO-ND 0.1-5 had increased non-flammability (V-1 rating) with GO-ND loading. GO-ND loading also showed electrical conductivity improvement (1.8-2.5 Scm-1) relative to PVBVA/PS/GO materials (10-2-1.3 Scm-1).
Keywords: Graphene oxide-nanodiamond, Nano-bifiller, Polystyrene, Tensile strength, UL 94
Cite this paper: Ayesha Kausar, Formation and Properties of Poly (vinyl butyral-co-vinyl alcohol-co-vinyl acetate) / Polystyrene Composites Reinforced with Graphene Oxide-Nanodiamond, American Journal of Polymer Science, Vol. 4 No. 2, 2014, pp. 54-62. doi: 10.5923/j.ajps.20140402.05.
Article Outline
1. Introduction
- Polymeric nanocomposites have been primed using variety of polymer matrices (thermaoplastics, thermosetting polymers, etc.) as well as various nanofillers including carbon-based nanomaterials [1]. These nanocomposites demonstrate notable enhancement in physical characteristics such as mechanical, thermal and gas-barrier properties with the inclusion of small amount of nanofillers compared with the pristine polymers and conventional nanocomposites [2, 3]. In recent decades, range of carbon-based nanomaterials such as graphene, graphene oxide (GO) and nanodiamond (ND) have gained considerable attention owing to the outstanding physical properties. Nanodiamond has been produced and employed in different materials due to its tremendous worth in new carbon-based nanomaterial [4-6]. ND is known to have high dispersibility in polymer matrices and the resulting nanocomposites had superb physical properties, which can approach the values of single-crystal diamond [7]. Graphene, another type of nanocarbon, is two-dimensional sheet of covalently bonded sp2 carbon atoms. Tremendous research has been carried out regarding graphene-based materials due to unique structure and properties [8]. Graphene oxide (GO) is an important functionalized graphene material which have oxygen-containing functional groups on basal planes and edges of graphene [9]. These groups render the preferred characteristics for the dispersibility of the sheets in matrix. Since GO is chemically analogous to carbon nanotube and structurally akin to layered clay it has a great latent to concurrently advance not only the mechanical and barrier properties but also functional properties like electrical and thermal conductivity of polymers [10, 11]. Despite the potential advantages, the synthesis of GO-reinforced polymer nanocomposites has been challenging in obtaining well-dispersed GO sheets in polymer matrix. Several nanocomposites have been prepared with well-dispersion in polymer matrices and overcome the drawbacks of the conventional polymer/carbon-based materials. Generally, solution mixing has been used to form polymer/GO nanocomposites. Poly(methyl methacrylate) (PMMA), polyacrylic acid (PAA), polyacrylonitrile (PAN), etc. have been successfully applied in nanocomposite fabrication with GO using solution processing [12, 13]. New flame retardant approaches for polymer materials have been developed using nanocarbon additives [14–16]. In this regard many research efforts have focused on graphene, carbon nanotubes, carbon nanofibers, etc. [17-19]. However, to use of these nanoadditives as efficient flame retardants need to overcome the aggregation of nanoparticles and increase the resin viscosity at high loading levels [20-23]. In this research, we have chosen a blend system of poly(vinyl butyral-co-vinyl alcohol-co-vinyl acetate) (PVBVA) and polystyrene (PS). Firstly, we have opted PVBVA as a matrix component due to the fact that its exploitation as a matrix (especially to enhance the electrical conductivity) is relatively less explored in literature. By far the most extensive use for PVBVA is in automotive and architectural applications. The polymer is also employed in coatings, binders, primers, and toners. However, the PVBVA has a tendency to crosslink which may deteriorate the final nanocomposite properties. Therefore, polystyrene was added as matrix component to improve the hybrid properties by influencing the crosslinking behavior of PVBVA. Consequently, PVBVA and PS-based nanocomposites have been developed using two type of nanocarbon structures. For this purpose, nanodiamond- functional graphene oxide (GO-ND) has been developed and both the graphene oxide and nanodiamond-functional graphene oxide were employed as nanofiller. GO-ND and ND-reinforced PVBVA/PS nanocomposites were prepared by simple casting method. The structure and properties of PVBVA/PS/GO and PVBVA/PS/GO-ND nanocomposites reinforced with GO and GO-ND were investigated using Fourier transform infrared spectroscopy (FTIR), field emission scanning electron microscopy (FESEM), thermogravimetric analysis (TGA), tensile tests, flammability and electrical conductivity.
2. Experimental Section
2.1. Materials
- Poly(vinyl butyral-co-vinyl alcohol-co-vinyl acetate) (average Mw = 120,000) and polystyrene (average Mw ~ 350,000, average Mn ~ 170,000) were purchased from Aldrich. Detonation nanodiamond powder with size in the range of 4-5 nm was obtained from ITC, USA. Graphite was provided by Asbury Carbons, USA. Nitric acid (90%, HNO3), sulfuric acid (98%, H2SO4), hydrochloric acid (37%, HCl), potassium persulfate (99 %, K2S2O8), and phosphorus pentoxide (99%, P2O5) were bought from Aldrich and were used without further purification.
2.2. Instrumentation
- Fourier transform infrared (FTIR) spectra were recorded using Excalibur Series FTIR Spectrometer, Model No. FTSW 300 MX manufactured by BIO-RAD. Stress-strain response of the samples (strips) with (ca. 15×5.6-9.0×0.45 – 0.77 mm) dimensions was monitored according to DIN procedure 53455 using Universal Testing Machine INSTRON. Standard procedures and formulae were used to calculate stress, strain, young’s modulus and toughness. The samples were cryogenically fractured in liquid nitrogen for phase morphological studies using FEI Nova 230 field emission scanning electron microscope (FE-SEM). Thermal stability of the nanocomposites was determined by NETZSCH thermo gravimetric analyzer (TGA), model no. TG 209 F3, using 1-5 mg of the sample in Al2O3 crucible from upto 800°C at a heating rate of 10°C/min under nitrogen flow rate of 30 mL/min. LOI value was measured on samples (100×5.8×3 mm3) according to the standard oxygen index test ASTM D2863-77 using FTA IL. UL-94 test was performed (129×15.6×3 mm3) according to ASTM D635-77 for UL-94 test. Electrical conductivity was measured with four probe technique in order to avoid the contact resistance. A constant current was applied using Keithley 2400 source meter to the outer probes of the four contacts and corresponding voltage was measured with Keithley 2182 nanovoltmeter between inner probes at room temperature.
2.3. Preparation of Graphene Oxide (GO)
- The graphite powder (15 g) was put into a solution of concentrated H2SO4 (60 mL), K2S2O8 (7.8 g), and P2O5 (8.4 g) and refluxed at 80°C. After 2 h, the mixture was left to cool down to room temperature for 6 h. The mixture was then carefully diluted with 300 mL of distilled water, filtered, and washed until the pH became neutral. The product was dried at 60 °C for 48 h [24].
2.4. Synthesis of Carboxylated Nanodiamond (ND-COOH)
- Nanodiamond powder (0.5g) was heated in 9:1 (v/v) mixture of concentrated H2SO4 and HNO3 (30 mL) at 80°C for 24 h. 0.1 M NaOH aqueous solution (50 mL) was added to the above mixture and was refluxed at 90°C for 2h. Afterwards, 0.1 M HCl aqueous solution (20 mL) was added and refluxed for 2 h at 90°C. The resulting oxidized nanodiamonds were centrifuged for 6 h, filtered and washed until the pH was neutral. The product was dried at 70°C [25].
2.5. Graphene Oxide-nanodiamond Synthesis (GO-ND)
- Into a 500 mL round bottom flask, GO (0.6g) and ND-COOH (0.2 g) were sonicated in 50 mL of deionized water for 6h to achieve homogeneous dispersion. Afterwards, 300 mL HNO3 was added to the above mixture of GO and ND-COOH. The sample was again sonicated for 4 h at room temperature. After the desired time, the mixture was centrifuged for 4 h. The resulting GO-ND hybrid was washed with deionized water and filtered. Finally, the product was dried at 70°C.
2.6. Preparation of Poly(vinyl butyral-co-vinyl alcohol-co-vinyl acetate)(PVBVA)/GO-ND Nanocomposites
- Typical procedure for the formation of well-dispersed PVBVA/PS/GO-ND nanocomposite includes the dispersion of GO-ND powder (0.1 g) in THF (20 mL) with sonication of 6h at room temperature. The polymer matrix was comprised of 1:1 wt. % of PVBVA: PS. Subsequently, the solution of 1 g of PS and PVBVA was separately prepared in 10 mL THF and was added to the above GO-ND suspension. The mixture was further sonicated for 4 h to yield a stable suspension. Finally, the homogeneous PVBVA/PS/GO-ND solution was poured into a Teflon Petri dish and kept at 60°C for film casting. Similarly, PVBVA / PS/GO-ND nanocomposite films (Fig. 1) were prepared using same procedure with loadings 0.1, 0.5, 1 and 5 wt. %, respectively, as well as one sample of pristine PVBVA/PS was also prepared.
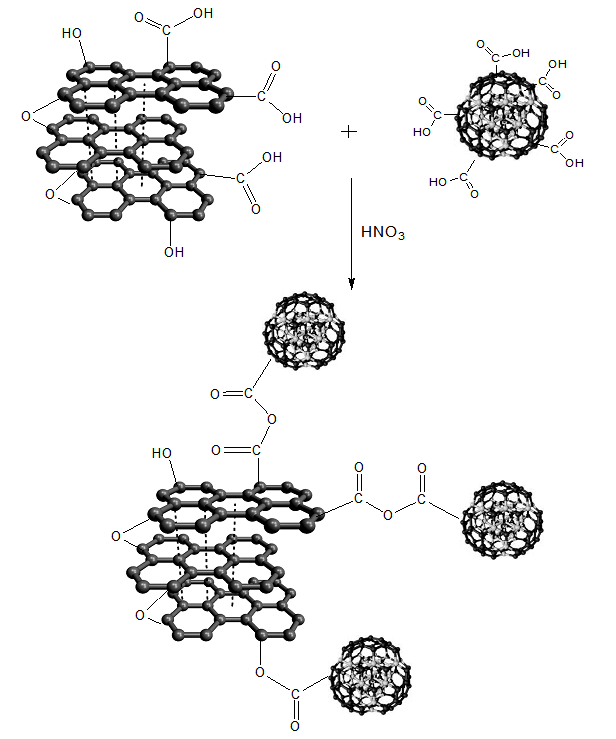 | Scheme 1. Formation of graphene oxide/nanodiamond (GO-ND) nano-bifiller |
 | Figure 1. Films of (A) PVBVA/PS/GO 0.5; (B) PVBVA/PS/GO-ND 0.5 |
3. Results and Discussion
3.1. FTIR Analysis
- FTIR spectra of GO, ND-COOH and GO-ND are plotted in Fig. 2.
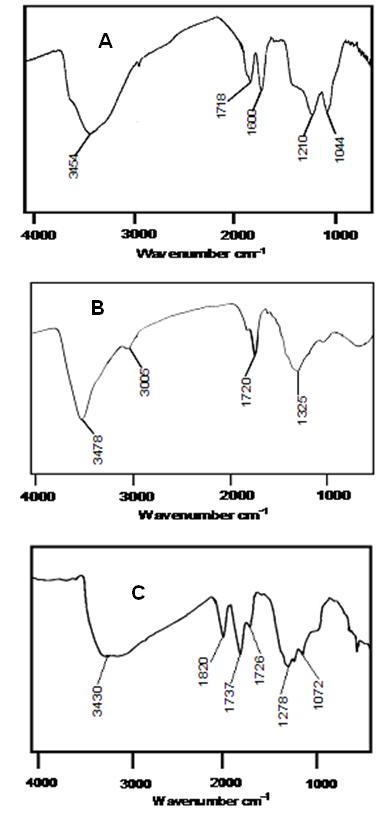 | Figure 2. FTIR spectra of (A) graphene oxide; (B) carboxylated nanodiamond; and (C) GO-ND filler |
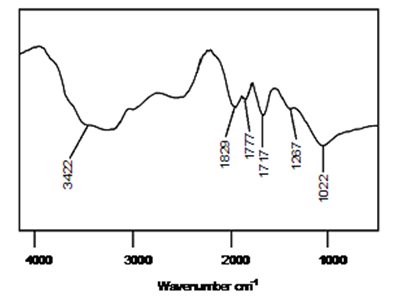 | Figure 3. FTIR spectrum of PVBVA/PS/GO-ND 0.5 |
3.2. Morphology Investigation
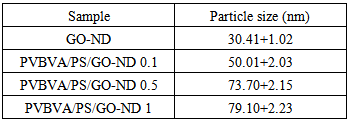
- The morphology of as-prepared GO, GO-ND, PVBVA/PS/GO 0.1-5 and PVBVA/PS/GO-ND 0.1-5 were investigated by field emission scanning electron microscopy. As the interaction of nano-bifillers to matrix could strengthen the interface interaction and consequently result in fine dispersion that largely determines the performance of nanocomposites. Fig. 4A shows the FESEM image of graphene oxide sheet while Fig. 4B depicts the formation of GO-ND structure. Exfoliated GO sheets embedded with nanodiamonds were visible in nano-bifiller morphology. The morphology of PVBVA/PS/GO 0.1 is given in Fig. 4C-E. The PVBVA/PS/GO-ND 0.1 shows homogenously dispersed GO-ND in PVBVA/PS matrix and no obvious aggregation was observed in the sample. However, in Fig. 4E at higher resolution polymer coating was observed over the surface of dispersed GO-ND nano-bifiller. Moreover, no sacked structure of GO sheets was experiential due to good dispersion of GO-ND in matrix. In the case of PVBVA/PS/GO-ND nanocomposite with 0.5 wt. % filler (Fig. 5A & B), dispersion was even better relative to PVBVA/PS/GO-ND 0.1 sample. Here again the GO sheets with NDs were coated with PVBVA/PS matrix. The dispersion of 0.5 wt. % GO-NDs was better compared to other nanocomposites prepared. Fig. 5C & D represent the morphology of PVBVA/PS/GO-ND 1. Some aggregation of the nano-bifiller was visible in the matrix with 1 wt. % loading, illustrating the lack of uniform dispersion using higher filler content. The particle size measurement of nano-filler and hybrids is given in Table 1. According to the results the least particle size was obtained for GO-ND around 30.41+1.02 nm. Whereas, the nanocomposites have increased particle size in the range of 50.01-79.10 nm due to the deposition of polymer over the nano-filler surface.
|
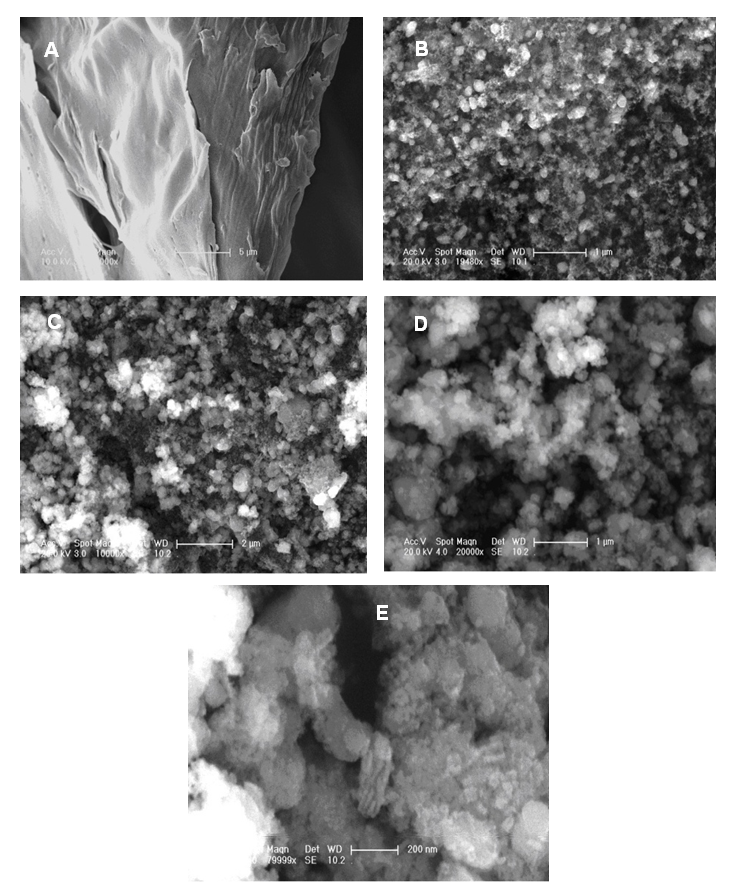 | Figure 4. FESEM images of (A) GO; (B) GO-ND; (C) PVBVA/PS/GO-ND 0.1 at 2 µm; (D) PVBVA/PS/GO-ND 0.1 at 1 µm; (E) PVBVA/PS/GO-ND 0.1 at 200 nm |
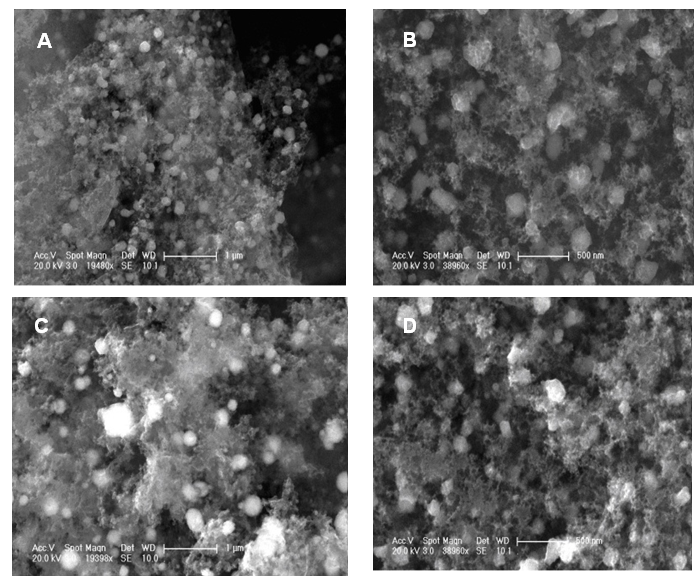 | Figure 5. FESEM images of (A) PVBVA/PS/GO-ND 0.5 at 1 µm; (B) PVBVA/PS/GO-ND 0.5 at 500 nm; (C) PVBVA/PS/GO-ND 1 at 1 µm; (D) PVBVA/PS/GO-ND 1 at 500 nm |
3.3. Tensile Studies
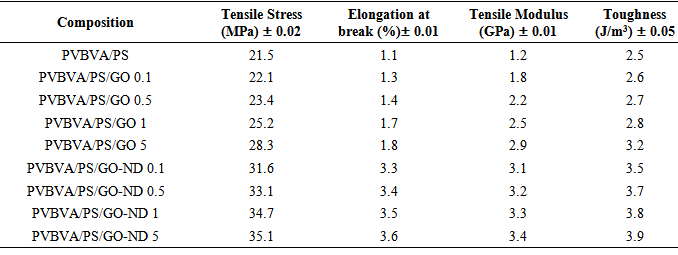
- Tensile properties of PVBVA/PS, PVBVA/PS/GO 0.1-5 and PVBVA/PS/GO-ND 0.1-5 nanocomposites are given in Table 2. Neat PVBVA/PS demonstrated tensile strength, modulus and toughness of 21.5 MPa, 1.2 GPa and 2.5 J/m3. Comparing to the neat PVBVA/PS blend, PVBVA/PS/GO 0.1-5 nanocomposites showed increase in tensile strength and modulus with the graphene oxide loading. Both PVBVA/PS/GO 0.1 and PVBVA/PS/GO 0.5 nanocomposites depicted increase in tensile modulus and strength. Tensile modulus was increased up to 1.3 and 1.4 GPa in PVBVA/PS/GO 0.1 and PVBVA/PS/GO 0.5 respectively. Similarly, tensile stress was raised to 22.1 and 23.4 MPa. Toughness of the samples was also found to enhance up to 2.6 and 2.7 J/m3. Somewhat better results were observed for the 5 wt. % GO loaded sample, which showed an increase of 41 % in tensile modulus and 34 % in strength compared to the neat blend. Typical tensile stress-strain behavior of PVBVA/PS/GO-ND 0.1-5 nanocomposites is shown in Fig. 6. The 0.1 wt. % GO-ND nano-bifiller exhibited substantial augment in both strength and modulus up to 31.6 MPa and 3.1 GPa, compared to pure blend and GO reinforced films. The 0.5 wt. % GO-ND nanocomposite showed slight increase in properties up to 33.1 MPa and 3.2 GPa. Both of the PVBVA/PS/GO-ND samples exhibited significantly higher toughness values (3.5 and 3.7 J/m3) compared with neat blend and GO composites. The nanocomposites with higher GO-ND loading revealed further higher values in tensile properties. PVBVA/PS/ GO-ND 1 had the tensile strength of 34.7 MPa and modulus of 3.3 GPa. PVBVA/PS/GO-ND 5 showed higher values among all the nanocomposites prepared with tensile strength of 35.1 MPa and modulus of 3.4 GPa. Toughness and strain values for this sample were also found to be higher around 3.9 J/m3 and 3.6 %.
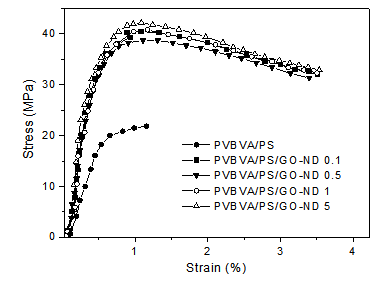 | Figure 6. Stress-strain curves of PVBVA/PS/GO-ND 0.1-5 nanocomposites |
|
3.4. Thermogravimetric Analysis
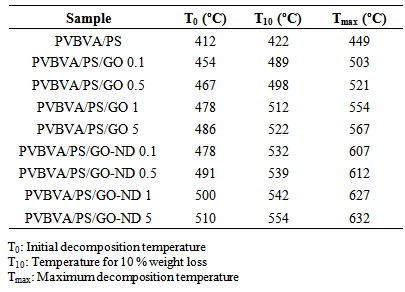
- Thermal stability of graphene oxide-based nanocomposites is another property that may be enhanced by the incorporation of GO sheets [26]. TGA was, therefore, employed to study the thermal degradation of nanocomposites under nitrogen atmosphere. Thermal data obtained from thermogravimetric analysis of PVBVA/PS, PVBVA/PS/GO 0.1-5 and PVBVA/PS/GO-ND 0.1-5 nanocomposites are presented in Table 3. At low GO content of 0.1 wt. %, the onset degradation temperature (T0) of the nanocomposite was improved to 454°C relative to PVBVA/PS (412°C). This was due to the homogeneously distributed and well exfoliated GO sheets which may hinder the production of thermally degraded volatile product causing the degradation. Similarly, 10 % degradation temperature (T10) of PVBVA/PS/GO 0.1 was increased to 489 ºC and maximum decomposition temperature (Tmax) was found at 503 ºC relative to PVBVA/PS (T0 = 422 ºC; T10 = 449 ºC). Thermal stability of PVBVA/PS/GO 5 was found to be further increased to T0 = 486 ºC; T10 = 522 ºC; Tmax = 567 ºC with 5 wt. % filler loading. Fig. 7 shows that the GO-ND sheets filled nanocomposites have significant improvement in thermal stability compared with neat PVBVA/PS and PVBVA/PS/GO 0.1-5. The thermograms exposed two-step thermal degradation profile. Among PVBVA/PS/GO 0.1-5 materials, PVBVA/PS/GO-ND 0.1 with 0.1 wt. % nano-bifiller had T0 of 478 ºC, T10 of 532ºC and Tmax of 607ºC. Adding up 0.5 wt. % filler in PVBVA/ PS matrix further enhanced the heat constancy to T0, T10 and Tmax of 491ºC, 539ºC and 612ºC. PVBVA/PS/ GO-ND 5 exhibited highest values of T0 = 510 ºC, T10 = 554ºC and Tmax = 632ºC among these nanocomposites. The graphene oxide-nanodiamond nano-bifiller infact had improved dispersion in the matrix ensuing unique morphology and enhanced thermal stability.
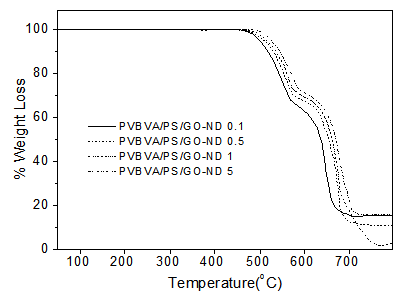 | Figure 7. TGA curves of PVBVA/PS/GO-ND 0.1-5 nanocomposites at 10 oC/min (N2) |
3.5. Flammability Tests
|
|
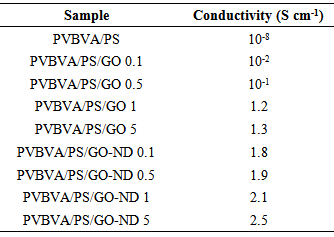
3.6. Electrical Conductivity Measurement
- Although the reported electrical conductivity of graphene oxide has been known much lower than graphene, the graphene oxide and nanodiamond nano-bifiller was expected to form conducting network supporting the increased electrical conductivity. The electrical conductivity values for PVBVA/PS/GO 0.1-5 and PVBVA/PS/GO-ND 0.1-5 nanocomposites are listed in Table 5. The electrical conductivity of PVBVA/PS/GO 0.1-5 nanocomposite films was found in the range 10-2-1.3 Scm-1. On the other hand, PVBVA/PS/GO-ND 0.1-5 nanocomposites had higher values in the electrical conductivity 1.8-2.5 Scm-1. Generally speaking, rapid increase in the electrical conductivity of GO-ND-based nanocomposites implies the formation of a conducting network throughout the insulating polymer matrix [28, 29]. However, PVBVA/PS/ GO 0.1-5 nanocomposites were still sufficiently electrically conductive, which may also be attributed to the network between PVBVA/PS matrices and GO generating some conductive pathways in the nanocomposites.
|
4. Conclusions
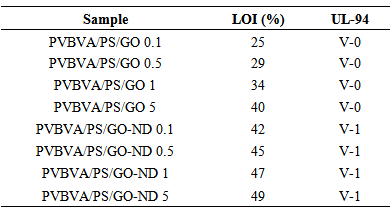
- Graphene oxide was directly functionalized with nanodiamonds to form nano-bifiller. The GO and GO-ND werer afterwards introduced into PVBVA/PS blend structure to obtain PVBVA/PS/GO and PVBVA/PS/ GO-ND nanocomposites. GO-ND sheets were dispersed well in matrices without any reaggregation, and they were partly covered with polymers. The nanocomposites exhibited a significant improvement in mechanical property and thermal stability at GO and GO-ND loading level of 0.1-5 wt. %. As the functionalization of GO with ND affords a facile and cost-effective way to prepare nanodiamond decorated graphene oxide and PVBVA / PS-based nanocomposites with high performance. Nano-dispersion of functional graphene oxide also resulted in non-flammable nanocomposite with enhanced electrical, thermal, mechanical and morphological properties. One of the fine achievements was the accomplishment of LOI values between 42-49 % and V-1 rating of PVBVA/PS / GO-ND 0.1-5. Moreover nanodiamond in combination with GO revealed better flame retardant effects. Conductivity measurements performed highlighted that the nano-diamond filler enhanced the conductive properties. Novel materials can be engineered into thermal spray coatings to provide different degrees of electrical conductivity.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML