-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Polymer Science
p-ISSN: 2163-1344 e-ISSN: 2163-1352
2014; 4(2): 46-53
doi:10.5923/j.ajps.20140402.04
Bacterial Cellulose and Its Phosphonic Dichloride for Efficient Removal of Metal Ions
Sherif M. A. S. Keshk1, 2, Mohamed S. Omar3, 4
1King Khalid University, Faculty of Science, Chemistry Department, P.O. Box 9004, Abha 61413- Saudi Arabia
2Ain Shams University, Institute of Environmental Studies and Research, Basic Science Department, Abbassia, Cairo 11566, Egypt
3Benha University, Faculty of Science, Chemistry Department, Qalyubia, Egypt
4Taif University, College of Pharmacy, Pharmacology and Toxicology Department, Taif, Saudi Arabia
Correspondence to: Sherif M. A. S. Keshk, King Khalid University, Faculty of Science, Chemistry Department, P.O. Box 9004, Abha 61413- Saudi Arabia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Bacterial cellulose (BC) in comparison with cotton stalks (CS) and their phosphonic dichloride derivatives for efficient removal of metal ions were investigated. BC and its phosphonic dichloride have the highest efficiency toward metal ion uptake, due to its low degree of polymerization and the largest surface area. FT-IR results showed two bands at 1198 cm-1 and 960 cm-1 that characteristic to C-O-P bond. Furthermore, the fragments obtained from mass spectrum was characteristic to six carbon sugar mono phosphonic dichloride for both BC and CS. X-ray diffractgram confirmed the presence of phosphorous group at 2θ=30ᵒ that corresponding to (211) plane.
Keywords: Bacterial cellulose, Cotton stalks, Phosphonic dichloride, Degree of polymerization
Cite this paper: Sherif M. A. S. Keshk, Mohamed S. Omar, Bacterial Cellulose and Its Phosphonic Dichloride for Efficient Removal of Metal Ions, American Journal of Polymer Science, Vol. 4 No. 2, 2014, pp. 46-53. doi: 10.5923/j.ajps.20140402.04.
Article Outline
1. Introduction
- The consumption of disposable materials has increased and hence, the load on the environment has increased dramatically. Consequently, the demand for bio-based materials with renewable, low-cost and sustainable properties has increased. Cellulose-based materials can potentially provide products to meet this demand. Annual cellulose synthesis by plants is close to 3x1012 tons (Klemm et al, 1998). Plants contain approximately 33% cellulose, whereas, wood contains around 50% and cotton contains 90%. Cotton and wood are the major resources for all cellulose products such as paper, textiles, construction materials, and cardboard, in addition to cellulose derivatives such as cellophane, rayon, and cellulose acetate (Colvin, 1980). Unlike the cellulose from wood pulp, cellulose produced by an Acetobacter strain is devoid of other contaminating polysaccharides and its isolation and purification are relatively simple, not requiring energy- or chemical-intensive processes. Furthermore, environmental problems due to by-products of wood pulping given an added impetus to study unexplored sources of cellulose (Keshk, 2014 a, b). Bacterial cellulose (BC) generated as a never-dried membrane from static culture in a nearly pure form that contains 99.1wt% water of which 0.3wt% is bound and 98.8wt% is free of water (Brown et al., 1976). It has more than 200 times greater surface area than isolated softwood cellulose and has a tensile strength similar to that of steel (Delmer & Yehudit, 1995). BC is excellent adsorbents for organic dyes, due to their fibrous structure and high hydrophilic sorption capacity (Delmer & Yehudit, 1995). Cellulose can be chemically modified to yield derivatives which are used widely in different industrial sectors in addition to conventional applications. As an example, cellulose was used as a raw material in the production of regenerated fibres and films in addition to cellulose derivatives (Klemm et al, 1998). Derivatives are further used as coatings, laminations, optical films and absorbents. Additionally, cellulose derivatives can be found as additives in building materials and also in pharmaceutical, food and cosmetic products (Klemm et al, 1998; Keshk & Hajia, 2011). The reactivity and availability of the hydroxyl groups of BC have been investigated via chemical reactions as well as the subsequent characterization of the derivatives obtained (Keshk & Nada, 2003; Keshk, 2008, 2011, 2014a). BC is more reactive than other natural cellulose towered homogenous and heterogeneous reactions owing to increased number of the hydroxyl groups in BC chains in addition to its lower degree of polymerization than those of natural cellulose (Keshk, 2008; Keshk & Nada, 2003). The removal of heavy metal ions from aquatic systems is the subject of extensive technological research and recovering processes. Various methods have been reported for the treatment of aqueous streams contaminated with heavy metals, among them polymers (Rivas, 1998; Zhou et al., 2004; Okieimen et al., 2005; Keshk & Nada, 2008) amorphous silica (Padilha et al., 1999; Goswami & Singh, 2002), clays (Mercier & Detellier, 1995; Mercier & Pinnavaia, 1998, Hasine et al., 2008) and zeolites (Ahmed et al., 1998; Erdem et al., 2004). Nevertheless, these materials have shown several problems like low mechanical and thermal stability, weak chemical labeling with the metals, poor removal efficiency, high cost etc. The effective and economic methods are based on adsorption process, so the synthesis of adsorbents for the removal of toxic heavy metal ions from waste water is a continuing research objective of environmental pollution control processes. In this work, the efficiencies of BC and CS to remove heavy metal ions (Cr3+, Fe3+, Cu2+, Zn2+ and Pb2+) from aquatic systems in comparison with phosphonic dichloride derivatives of both BC and CS will be evaluated. Furthermore the chemical structure of phosphonated cellulose will be discussed using FT-IR, mass spectra and X-ray diffraction.
2. Materials & Methods
- The raw materials used in this study were CS (DP = 1250) and BC (DP 890). American type culture collection (ATCC) is the supplier of the Gluconacetobacter xylinus. The chemicals used throughout this work were purchased from Sigma and Aldrich Chemical Co.
2.1. Culture Media and Conditions
- The culture medium is namely the modified Hestrin-Schramm culture medium in presence of 0.5% Ascorbic acid (Keshk, 2014b). The culture was incubated statically at 28C in the liquid medium at pH 6 for one week.
2.2. Pellicles Production and Purification
- BC membrane was produced by different Gluconacetobacter xylinus strains in 30 ml of HS and HSA media at 28C for 7 days using 90-mm (i.d.) petridishes. After incubation, the pellicles produced on the surface of each medium were harvested and washed with water, hot 2% sodium dodecyl sulfate (SDS) and water successively, then dried on a Teflon plate.
2.3. Extraction of Cellulose from Cotton Stalks
- Cotton stalks were ground by hammer mill. The ground CS was refluxed with 6% sodium hydroxide for two hours. The produced pulp was bleached with hypochlorite (3%) in two stages for 1.5 hrs at 60°C then filter and washed till neutralization.
2.4. Mercerization of Cellulose
- Cellulose samples were soaked in 20% NaOH for 48 hrs and then washed with deionized water well till neutralization.
2.5. Preparation of Cellulose Phosphonic Dichloride
- Mungall et al (1974) preparation procedure was modified as follow: 1.0g of dry mercerized BC or CS samples were suspended in cold dry pyridine (5C) for 12 hrs, and then add dropwise mix of 5 ml of phosphorus oxy-chloride in 25ml methylene chloride. The contents were heated for 2 hrs. Then, the contents were cooled and poured onto ice and filtered. The filtered was washed with 0.1N HCl, and deionized water till neutralization.
2.6. Determination of Phosphorus Content
- Cellulose phosphonic dichloride (0.2g) was boiled in conc. HNO3 till dissolution and then cooled and diluted to 100 ml with deionized water. Phosphorus was determined in the solution using ICP-AES Yvon J4185 spectrophotometer.
2.7. Determination of Metal Ion Uptake
- BC, CS and their phosphonic dichloride derivatives (0.2g) were stirred in metal ion (Cr+3, Fe+3, Cu+2, Zn+2 and Pb+2; 20μg of each) solutions (25ml), for 30 min. The mixture was filtered and the remaining metal ions in the filtrate were determined. Metal ion uptake and phosphorus was determined in the solution by using ICP-AES technique (Inductive coupled plasma-Atomic Emission spectrometry).
2.8. FT-IR Spectroscopy Measurement
- IR spectra were measured using a Nihon FT-IR-480 spectrometer. The reproducibility of the spectra was verified on three-sample preparation; from 64 to 100 scans were taken with a resolution of
2.9. Mass Spectral Measurement
- Mass spectrum of cellulose phosphonic dichloride was recorded on Finningan SSQ 7000 spectrometer.
2.10. X-ray Diffractometry
- The thick sample was prepared according to Kai & Keshk (1998) Diffractogram of the sample was recorded at room temperature with RIGAKU PRINT 2200V series using Ni-filtered
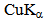 radiation (λ = 1.54 Å). The operating voltage and current were 40 Kv and 30 mA, respectively. Crystallinity index (C.I.) was calculated from the reflected intensity data using the Segal et al, method, according to Eq. (1)
radiation (λ = 1.54 Å). The operating voltage and current were 40 Kv and 30 mA, respectively. Crystallinity index (C.I.) was calculated from the reflected intensity data using the Segal et al, method, according to Eq. (1)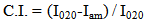 | (1) |
3. Results & Discussion
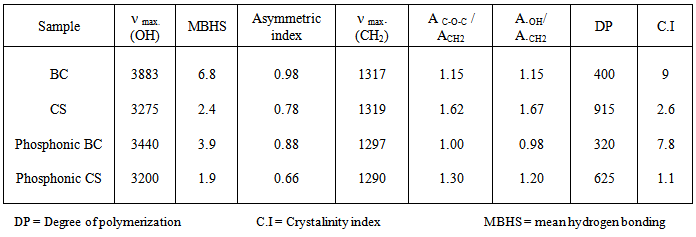
- Inferred spectra of BC and CS were recorded in the region of 4000 to 500 Cm-1. The relative absorbance of the subscript wave number to the absorbance of the wave number at 1328 Cm-1 which is corresponding to the CH rocking of the ring was calculated for samples (El-Sayed et al, 1997). The asymmetry index (It was defined as the ratio of the band width at 4000-3000 Cm-1 region) on the low and high frequency side of the maximum at half band absorbance the bonded hydroxyl stretching gives unsymmetrical band with broadening towards the lower frequency (Nada et al , 1990, 2000). On the other hand, the mean strength of the H-bonds (MBHS) was calculated as the ratio of AOH/ACH, where A. is the absorbance of the stretching vibration of subscript groups. Whereas the C.I. was calculated (the ratio of the absorbance of band at 1430 Cm-1 to band at 900 Cm-1) (Nada et al, 2000). Table (1) shows the maximum absorption band of stretching vibration of OH groups (AOH), MHBS, asymmetry index and C.I. of BC and CS. From Table (1), the asymmetry index reveals that the hydroxyl groups of the two kinds of cellulose are not free but entering into different modes of hydrogen bonds. Also, MHBS in case of BC is stronger than that in CS. This was confirmed by increasing in C.I. of BC rather than that in CS (Table 1). The relative absorbance of ether linkage (at 1120 Cm-1) in BC has the lower value than that of CS. This means that, BC has the lower degree of polymerization (DP) than that of CS. On the other hand, CS has the highest relative absorbance, revealing its high DP. The relative absorbance of primary OH at 1035Cm-1 of CS has a higher value than that of other kind of cellulose; it can be attributed to the higher DP than those of other two kinds (Table 1). These results are confirmed by measuring the DP as listed in Table (1). Figure (1) showed the heavy metal ions uptake by BC and CS. It is clear that, the metal ion uptake by BC was higher than that of CS. This may be attributed, to the highest porosity, hydrophilicity and the largest surface area of BC (Keshk, 2011). These phenomena of BC increase intrinsic absorption and columbic interaction of BC with heavy metals. The incorporation of phosphonic dichloride group onto BC or CS increases their efficiencies toward metal ions uptake (Figures 2, 3). This may be attributed to, the presence of phosphonic dichloride that facilitates chemical reaction of heavy metals with cellulose. BC has the highest phosphate content, where, BC has the lowest degree of polymerization that increases the reactive end groups of cellulose. The highest phosphate content in BC shows the lowest in relative absorbance of ether linkage in FT-IR spectrum, which means more degradation in ether linkage. The presence of pyridine in phosphonation process is important owing to its reaction with HCl that produced during phosphonation process. FT-IR of the incorporated phosphate groups onto BC recorded two new bands at wave lengths of 1198 and 960 Cm-1 those are related to C-O-P vibration band. Moreover, the quantity of incorporated phosphonic dichloride in BC is nearly related to the relative absorbance of these groups.
|
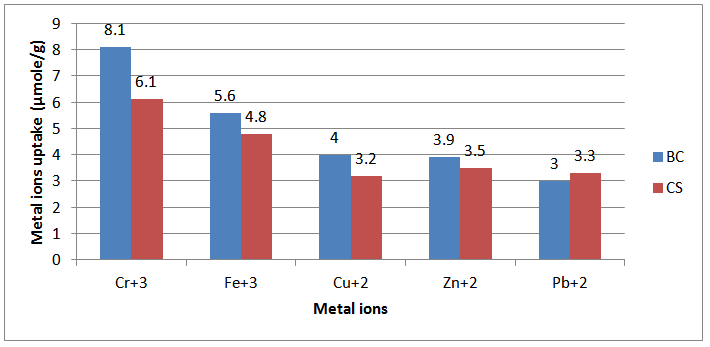 | Figure 1. Metal ions uptake (µmole/g) by Bacterial Cellulose (BC) and Cotton Stalks (CS) in 25 ml of metal ions solution |
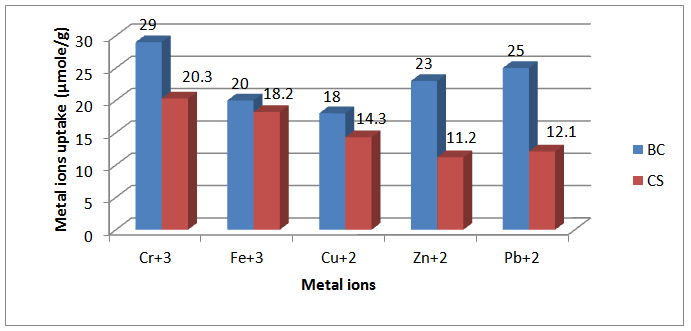 | Figure 2. Metal ions uptake (µmole/g) by Bacterial Cellulose (BC) and Cotton Stalk (CS) in presence of Pyridine |
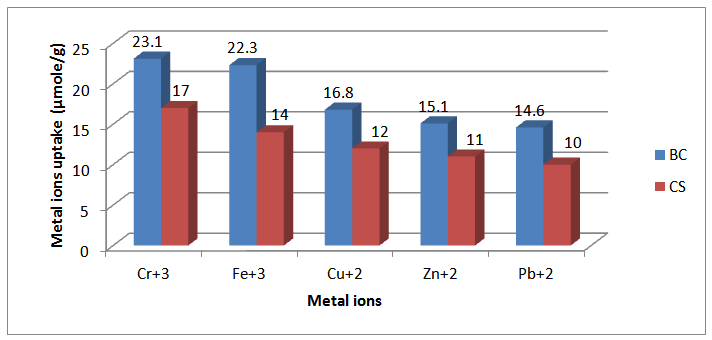 | Figure 3. Metal ions uptake (µmole/g) by Cellulose 6-Phosphonic dichloride of Both Bacterial Cellulose (BC) and Cotton Stalks (CS) |
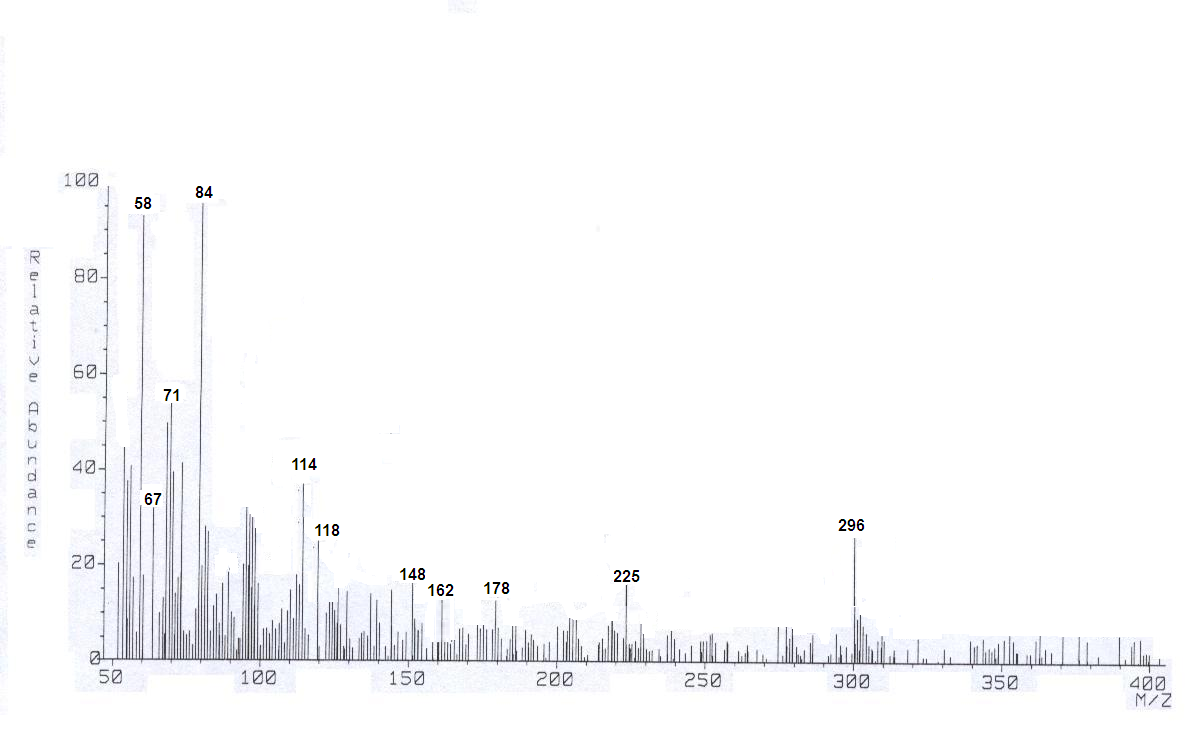 | Figure 4. Mass Spectra of Cellulose Phosphonic Dichloride |
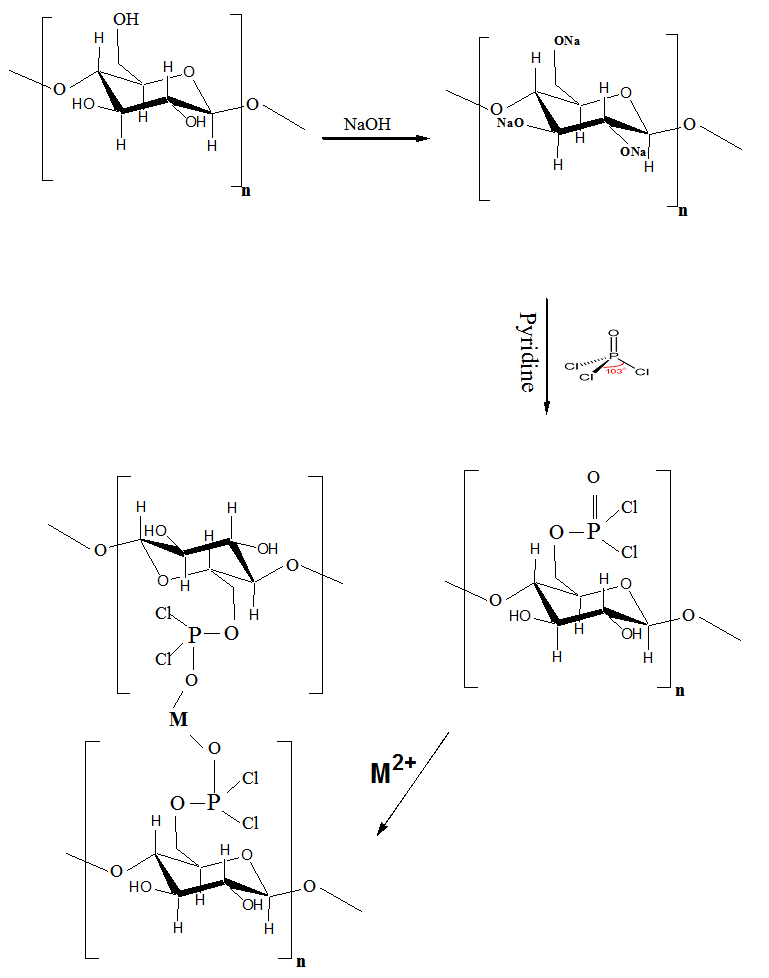 | Scheme 1. Reaction Mechanism of Cellulose 6-Phosphonic dichloride |
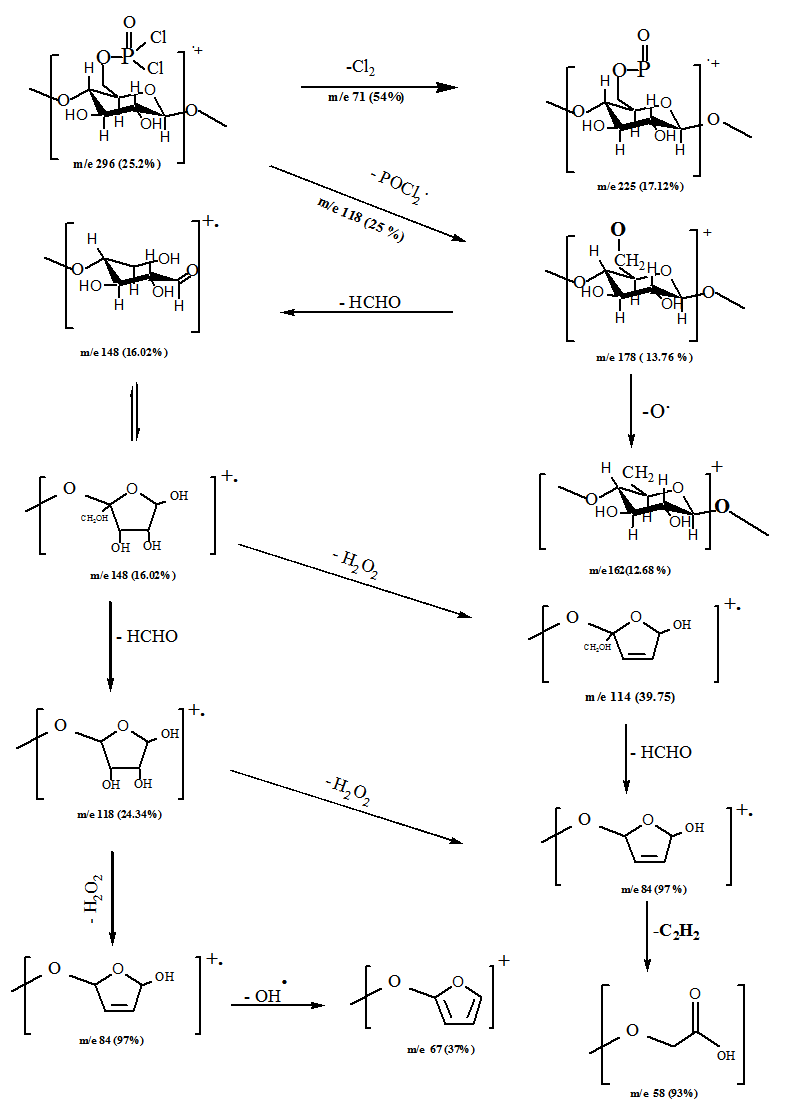 | Scheme 2. Fragmentation Pattern of the Cellulose 6- Phosphonic dichloride |
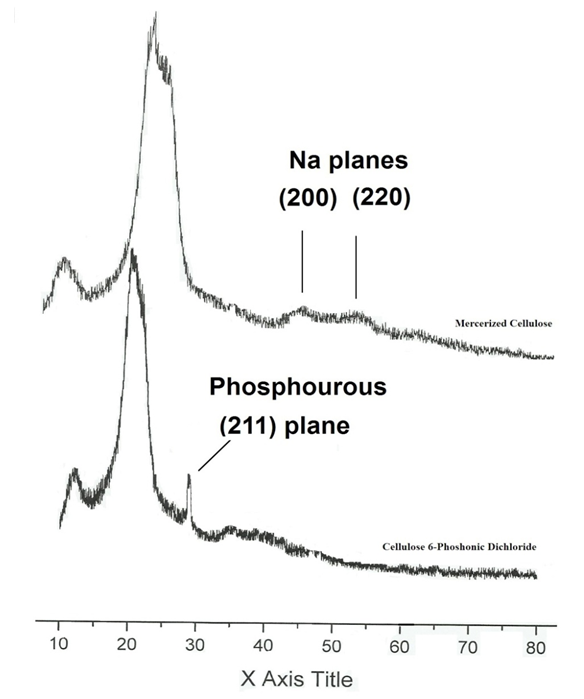 | Figure 5. X-ray Diffractogram of both Mercerized Cellulose and Cellulose 6-Phoshonic Dichloride |
4. Conclusions
- BC differs from cotton stalks with respect to its high crystallinity and purity from lignin. BC is more reactive towards phosphonation rather than that of CS owing to its high crystallinity (88%) with low degree of polymerization (890). Furthermore, BC has the highest ion exchange efficiency. Incorporation of phosphate group onto BC and CS increases their efficiency towards metal ion uptake.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML