Ergashev Nasriddin Shamsiddinovich , Raxmatullayev Adxam Abadbekovich
Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan
Correspondence to: Ergashev Nasriddin Shamsiddinovich , Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Background. Congenital malformations of the bronchopulmonary system (CMBPS), especially cystic forms, are a significant cause of mortality in children. While surgical intervention remains the primary treatment, the optimal timing and approach are still subjects of debate. Methods. This retrospective study analyzed the treatment outcomes of 115 children with cystic forms of CMBPS treated in the clinics of the Tashkent Pediatric Medical Institute from 2006 to 2022. Patients were divided into two groups: those managed using traditional methods (2006–2018) and those treated with modern diagnostic and surgical techniques, including videothoracoscopic surgery (2018–2022). Comparative analysis was conducted focusing on treatment approach, postoperative complications, and long-term outcomes. Results. The use of updated diagnostics and minimally invasive thoracoscopic surgery led to a reduction in unsatisfactory outcomes from 20% to 9%. Among all patients, 76.6% underwent surgery without complications, while 23.4% experienced postoperative issues such as pneumothorax, atelectasis, and infections. Minimally invasive techniques resulted in reduced surgical trauma, shorter hospital stays, and improved overall outcomes. Conclusion. Surgical resection of affected lung segments using minimally invasive technologies ensures better results in managing cystic bronchopulmonary malformations. Optimization of diagnostic and surgical strategies is especially effective in early childhood, supporting lung tissue regeneration and minimizing complications.
Keywords:
Cystic malformations of the bronchopulmonary system, Surgical treatment outcomes, Diagnosis, Treatment, Children
Cite this paper: Ergashev Nasriddin Shamsiddinovich , Raxmatullayev Adxam Abadbekovich , Immediate and Long-Term Results of Treatment of Cystic Forms of Bronchopulmonary Malformations in Children, American Journal of Medicine and Medical Sciences, Vol. 15 No. 5, 2025, pp. 1373-1378. doi: 10.5923/j.ajmms.20251505.12.
1. Introduction
According to the World Health Organization (WHO), congenital anomalies such as cardiac defects and pulmonary pathologies constitute a significant proportion of mortality causes among newborns and children under the age of five. Surgical correction of cystic forms of congenital malformations of the bronchopulmonary system (CMBPS) remains the only radical treatment method. The surgical approach in pediatric patients with various lung and mediastinal anomalies is determined based on the extent of the lesion and clinical manifestations.To date, there is no consensus on the optimal age for surgical intervention in children with pulmonary and mediastinal malformations. This issue continues to be widely debated in both domestic and international literature. Most of the described anomalies manifest later in the postnatal period, typically presenting with symptoms of respiratory insufficiency that arise due to complications such as compression of mediastinal structures or secondary infection.CMBPS is characterized by two contrasting courses: spontaneous regression of mild, asymptomatic forms, or progression into severe symptomatic forms with escalating clinical manifestations. Asymptomatic cysts pose a therapeutic dilemma—whether to opt for surgical intervention or conservative observation. In minimally symptomatic cases, a watchful waiting strategy is often justified [1,4,8,9,15]. It is evident that the best outcomes lie in a carefully considered combination of these approaches tailored to each individual case.It is also important to consider that lung tissue continues to grow through the formation of normal alveoli until the age of 5 to 8 years. Therefore, early surgical intervention offers a chance for complete recovery of lung volume and function through compensatory growth of healthy lung tissue [1,5,10,16].Objective of the study: To analyze the immediate and long-term outcomes of treatment for cystic bronchopulmonary malformations in children.
2. Materials and Methods
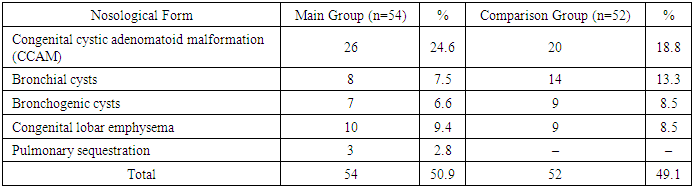
This study is based on the analysis of diagnostics and treatment outcomes in 115 children with congenital malformations of the bronchopulmonary system (CMBPS), who received treatment at the clinical bases of the Department of Pediatric Hospital Surgery of the Tashkent Pediatric Medical Institute between 2007 and 2022. Clinical sites included City Children's Clinical Hospital No. 2 (Tashkent), the Department of Neonatal Surgery at the Republican Perinatal Center of the Ministry of Health of Uzbekistan, and the Department of Minimally Invasive Pediatric Surgery of the National Children's Medical Center.The patients’ ages ranged from 1 day to 16 years, with the following distribution:• Newborns (up to 1 month): 31 patients (27%)• 1 month to 1 year: 29 patients (25.2%)• 1 to 3 years: 23 patients (20%)• 3 to 7 years: 13 patients (11.3%)• 7 to 15 years: 14 patients (12.2%)• 15 to 16 years: 5 patients (4.3%)The distribution of pathology varied depending on the patient's age, with the number of cases and types of diagnoses decreasing as age increased. Of the total 115 children observed, 47 (41%) were boys and 68 (59%) were girls.Among all patients, 106 (92.2%) were diagnosed with cystic forms of bronchopulmonary pathology, which form the focus of this study.Table 1. Nosological distribution of patients with cystic forms of CMBPS in the main and comparison groups (n = 106)
 |
| |
|
Patients were divided into two groups:• Comparison group (n = 52, 49.1%): treated between 2006 and 2017 using traditional diagnostic and surgical approaches.• Main group (n = 54, 50.9%): treated between 2018 and 2022 using an expanded diagnostic protocol, including prenatal screening of pregnant women and fetuses, as well as videothoracoscopic surgical interventions.
3. Results and Discussion
The presence of pronounced clinical signs of cystic forms of congenital bronchopulmonary malformations (CBPM) in the neonatal period, along with diagnostic imaging findings or the emergence of symptoms under aggravating conditions, indicates a severe disease course and necessitates surgical intervention.Surgical procedures were performed in 92 (86.8%) of 106 patients, with a total of 94 operations carried out. Two children with congenital cystic adenomatoid malformation (CCAM) required reoperations. Surgical interventions were performed in emergency, urgent-planned, or elective settings, depending on the patient’s condition and disease severity (see Table 2).Table 2. Treatment strategies for cystic bronchopulmonary anomalies (n = 106)
 |
| |
|
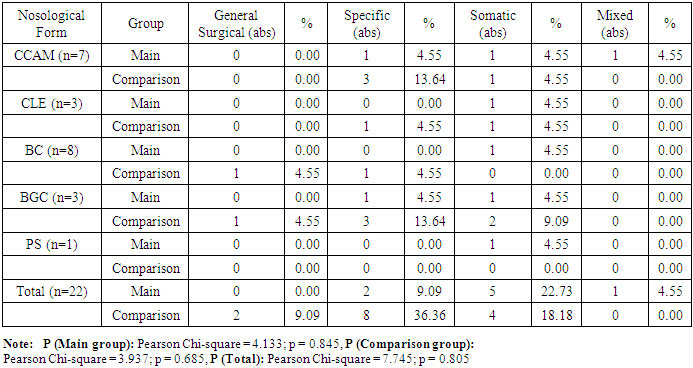
In 5 patients (4.7%) with asymptomatic or stable compensated disease, a watchful waiting strategy was deemed appropriate. These patients were monitored using dynamic functional evaluations to prevent potential complications. In 9 cases (8.5%), this conservative approach was chosen due to temporary refusal of surgery by the parents.The question of optimal timing for surgical correction remains controversial in the literature. Many authors recommend operative treatment regardless of clinical symptom severity, to avoid potential complications. In our observations, indications and timing for surgery were established individually for each patient, based on the severity and progression of clinical symptoms of the bronchopulmonary anomalies.The postoperative course was uneventful in 70 patients (76.1%). However, 22 patients (23.9%) experienced various complications. These were categorized as:• General surgical• Specific (e.g., pneumothorax, atelectasis)• Somatic (e.g., pneumonia, multiple organ failure)• Mixed-type (combinations of the above)(See Table 3 in the full article for detailed breakdown.) Table 3. Distribution of Observed Patients by Type of Complications (n = 22)
 |
| |
|
General surgical complications were observed in 2 cases (2.2%). Wound edge dehiscence was noted in one patient (1.1%) in the comparison group during the early postoperative period, and a ligature fistula was detected in one patient (1.2%) in the main group.Specific complications occurred in 10 patients (10.6%). These included pneumothorax (n=5), insufficient aerostasis (n=2), and lobar atelectasis (n=3). Specific complications were observed in 7 patients (7.4%) from the comparison group and in 3 patients (3.2%) from the main group.Somatic complications were found in 9 patients (9.6%), with 3 (33.3%) in the main group and 6 (66.7%) in the comparison group. These complications presented as pneumonia (n=8) and multiple organ failure (n=1) and primarily affected newborns born to mothers with unfavorable obstetric and gynecologic history, as well as perinatal and intranatal factors leading to fetal hypoxia.Among those who underwent emergency surgery in the early neonatal period:• 4 (44.4%) were operated on during the neonatal period,• 3 (3.2%) were under 3 months old,• 2 (2.1%) were under 3 years old.The most common specific and mixed complications included insufficient aerostasis, atelectasis of the remaining lobe, and multiple organ failure.A mixed complication—COVID-19 pneumonia complicated by multiple organ failure—was observed in 1 patient (1.1%) in the main group and resulted in death. In this case, three months after resection of the lingular segments of the left lung due to CCAM at the age of 1.5 months, recurrent cystic changes were found in the remaining parts of the lung, which were complicated by intrathoracic pressure syndrome. This necessitated a second operation—pneumonectomy of the affected side. On top of that, the child contracted COVID-19, which led to multiple organ failure and death.The fatal outcome was associated with the complexity of the congenital anomaly, the presence of concomitant diseases and anomalies, and technical and tactical errors at various stages of treatment.Of the 106 children with cystic forms of bronchopulmonary anomalies, 105 (99.1%) were discharged:• 91 (86.7%) after surgical treatment,• 14 (13.3%) after conservative management.Long-term outcomes were studied in 92 (87.6%) of the 105 discharged patients, with a follow-up period ranging from 6 months to 5 years. Among them:• 53 (57.6%) were from the main group,• 39 (42.4%) from the comparison group.Out of these, 82 patients (89.1%) were followed up after surgical treatment, and 10 patients (10.9%) were from the group managed with a watchful waiting approach.Patient complaints, physical examination findings, and additional diagnostic methods varied in character and severity.11 patients (13.1%) reported cough, varying in frequency (intermittent or constant) and nature (wet or dry), independent of the nosological form of the disease.In 8 cases (9.5%), emphysematous changes of varying degrees were noted in residual lung tissue on the operated side (see Figure 1a and 1b), without pronounced clinical signs but with a tendency toward frequent respiratory infections.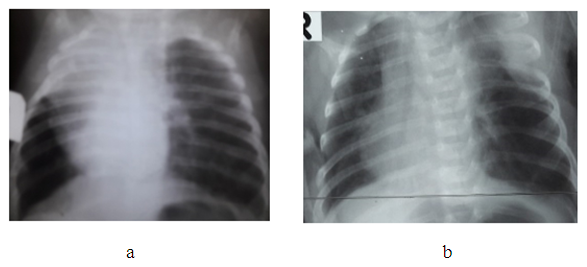 | Figure 1. (a) Emphysematous changes in the remaining lower lobe after right bilobectomy in a patient with lobar emphysema, patient U.Sh., 2 months old, case No. 67/47. (b) Emphysematous changes in the remaining lower lobe after left upper lobectomy, patient U.Sh., 1 month old, case No. 67/47 |
During dynamic observation, no progression in clinical or radiological findings was noted, which allows the emphysematous changes in the residual lung tissue after surgery to be considered compensatory in nature. However, the risk of underlying dysplastic changes cannot be ruled out. Continued follow-up of this patient will help determine an appropriate treatment strategy.In 3 patients (3.6%), long-term follow-up revealed chest wall depression of varying severity — from barely noticeable to clearly pronounced. This condition can be attributed to partial lung removal and/or the formation of atelectasis in the peripheral zones of resection, as well as to the development of adhesions and fibrous strands, causing compression of the lung parenchyma.Surgical outcomes were evaluated using a scoring scale and classified into good, satisfactory, and unsatisfactory results.A good outcome was observed in 52 cases (56.5%), characterized by:• No complaints or signs of respiratory insufficiency,• Absence of residual postoperative effects,• Symmetrical chest shape,• Good cosmetic appearance of the surgical scar (score: 5 points),• Normal chest X-ray or MSCT findings,• Pulmonary function tests within normal limits (score: 2–5 points) (see Fig. 2).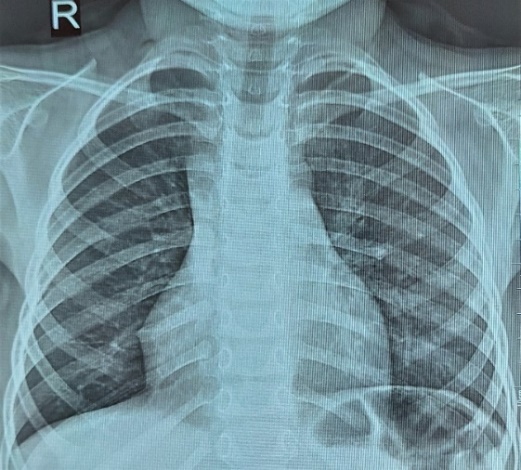 | Figure 2. Chest X-ray — full expansion of the remaining part of the lung after lobectomy for right-sided CCAM, patient I.S., 2 years 7 months old, case No. 3224-213 |
A satisfactory outcome was observed in 30 cases (32.6%). These patients reported cough in the absence of respiratory insufficiency. Moderate residual effects of the previous surgery were noted, requiring additional courses of conservative treatment.• There was mild chest asymmetry with partial chest wall depression.• The cosmetic appearance of the postoperative scar was considered satisfactory.• Imaging (X-ray or MSCT) showed partial lung expansion or localized atelectasis on the operated side (score: 6–9 points).• Pulmonary function tests showed a 15–30% reduction compared to normal values. (See Fig. 3.)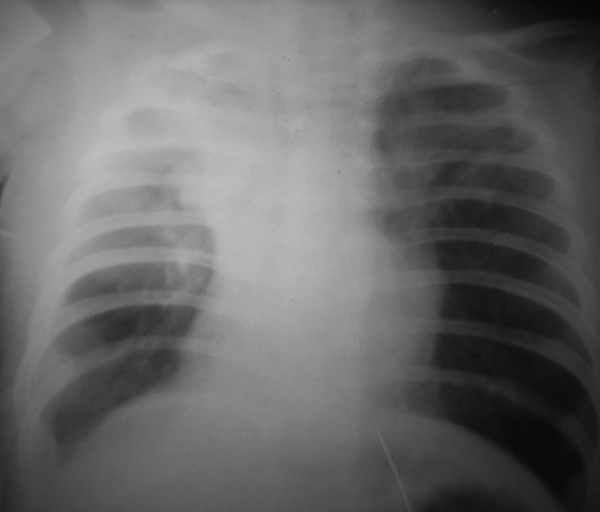 | Figure 3. Chest X-ray — partial expansion of the remaining part of the lung after lobectomy with partial atypical resection for right-sided CCAM, patient F.Sh., 1 year 1 month old, case No. 614-52 |
An unsatisfactory outcome was observed in 10 cases (10.9%). These patients reported:• Frequent coughing,• Episodes of respiratory infections,• Signs of respiratory insufficiency, which worsened with physical exertion.There were also residual effects of previous surgery that required repeat surgical intervention.• Additional findings included:• Pronounced chest asymmetry (depression or protrusion),• Surgical scars rated as satisfactory or keloid-like in appearance,• Imaging (X-ray or MSCT) showed partial lung expansion, marked emphysema, atelectasis, and pleural complications on the operated side.• Pulmonary function tests showed a greater than 30% reduction compared to normal values. (See Fig. 4.)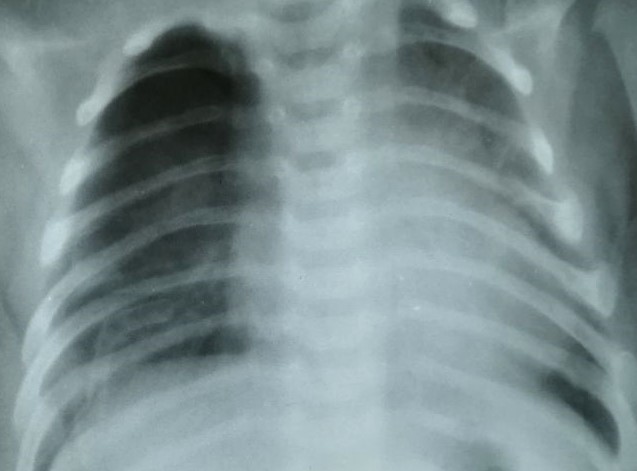 | Figure 4. Chest X-ray – pronounced emphysematous enlargement of the remaining lung segment after lobectomy with partial atypical resection for right-sided CCAM, patient Z., 2 years 7 months old, case No. 6231-1189 |
Follow-up examinations of 14 patients (16.7%) with cystic forms of bronchopulmonary malformations (10 with CCAM, 2 with BGC, 1 with BC, and 1 with PS) who exhibited mild and stable compensated clinical progression, and whose parents temporarily refused surgical treatment, were managed with a watchful waiting strategy.Over time, these patients showed a persistent and increasing tendency toward respiratory infections. In 5 of them (35.7%), the condition progressed from compensation to subcompensation, which became an indication for surgical intervention between 8 months and 2 years of age.Among 7 patients (18.4%) from the comparison group who underwent surgery after the age of 10 (3 with CCAM, 1 with BGC, 3 with BC) due to delayed diagnosis, the long-term outcomes revealed frequent episodes of chronic inflammatory diseases of the tracheobronchial tree. These negatively impacted the patients’ general somatic status and well-being. According to outcome criteria, 4 patients (4.3%) fell into the satisfactory category and 3 (3.3%) into the unsatisfactory category among the 92 patients assessed for long-term outcomes.Table 4. Long-Term Outcomes of Treatment of Cystic Forms of Bronchopulmonary Malformations Depending on Nosological Type in the Main and Comparison Groups (n = 92)
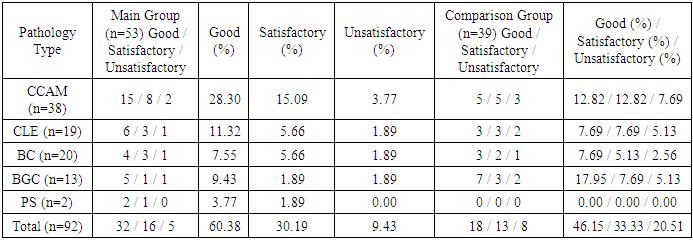 |
| |
|
As seen from these tables, patients in the main group demonstrated better outcomes. This can be attributed to the optimization of tactical and technical aspects of perioperative and postoperative management, as well as the implementation of high-informative diagnostic methods to identify anatomical features and functional disorders of the affected organs in bronchopulmonary anomalies (BPA).Among patients in the main group, the rate of unsatisfactory outcomes decreased from 20.5% to 9%, and the proportion of satisfactory outcomes decreased from 33.3% to 30.3%.
4. Conclusions
The results of this study indicate that in children with cystic forms of bronchopulmonary anomalies, properly implemented comprehensive postoperative care is a key factor in improving both short-term and long-term treatment outcomes.Improvement of tactical and technical approaches in surgical treatment and postoperative management of cystic BPA allowed for a reduction in the rate of unsatisfactory outcomes in the main group from 20.5% to 9%, and a reduction in satisfactory outcomes from 33.3% to 30.3%.The method of choice is the resection of the affected lung segments, with the use of thoracoscopic and video-assisted procedures, which significantly reduces surgical trauma, shortens hospitalization periods, and lowers treatment costs.The better results observed in the main group confirm the effectiveness of the developed diagnostic and operative-tactical approach for treating cystic forms of BPA in children. The study also showed that the relatively high incidence of certain complications—though not exceeding those reported in the literature—was due to complex clinical variants of the disease, delayed diagnosis, and concomitant conditions.
References
| [1] | Galyagina NA. Clinical and epidemiological characteristics and analysis of perinatal and long-term outcomes in children with congenital lung malformations [dissertation abstract]. Nizhny Novgorod; 2014. |
| [2] | Kokhno NI, Holmes NV. Congenital lung cysts. Zhurnal Natsional'nogo Mediko-Khirurgicheskogo Tsentra im. N.I. Pirogova. 2016; 11(2): 117-125. |
| [3] | Kozlov YA. Thoracoscopic approach in newborns: doctoral dissertation. Moscow; 2014. |
| [4] | Mashkov AE, Shcherbina VI, Stashuk GA, et al. Diagnosis and treatment of congenital cystic adenomatoid malformation in children. Detskaya Khirurgiya. 2017; 21(1): 23-27. |
| [5] | Patrikeeva TV. Congenital malformations of the lungs and mediastinum: diagnosis and treatment [dissertation abstract]. Saint Petersburg; 2016. |
| [6] | Razumovsky AYu, Sharipov AM, Bataev S-KhM, et al. Minimally invasive surgery in the treatment of children with congenital cystic adenomatoid lung malformation. Detskaya Khirurgiya. 2013; (2): 4-8. |
| [7] | Stepanenko NS. Thoracoscopic operations for lung malformations in newborns and infants [dissertation abstract]. Moscow; 2014. |
| [8] | Sharipov AM. Surgical treatment of congenital and acquired lung diseases in children [doctoral dissertation abstract]. Moscow; 2013. |
| [9] | Ergashev NSh, Rakhmatullaev AA. Congenital cystic adenomatoid malformation of the lungs: diagnosis and surgical tactics in children. Tibbiiyotda yangi kun. 2023; (8): 234-239. |
| [10] | Ergashev NSh, Rakhmatullaev AA. Bronchogenic and bronchial cysts in the structure of bronchopulmonary anomalies in children. Tibbiiyotda yangi kun. 2023; (10): 257-264. |
| [11] | Ergashev NSh, Rakhmatullaev AA. Lobar emphysema in the structure of bronchopulmonary developmental anomalies in children. Eur Asian J Pediatr. 2019; 3(3): 281-288. |
| [12] | Ergashev NSh, Rakhmatullaev AA. Lobar emphysema in the structure of cystic anomalies of the bronchopulmonary system in children. Sci Innov. 2023; 2(10): 56-63. |
| [13] | Arnaud D, Varon J, Surani S. An unusual presentation of congenital lobar emphysema. Case Rep Pulmonol. 2017; 2017: 6719617. doi:10.1155/2017/6719617 |
| [14] | Brown KL, Davis SM. MRI versus CT: optimal imaging modalities for pediatric bronchopulmonary cysts. Int J Pediatr Imaging. 2022; 17(1): 45-53. |
| [15] | Fainardi V, Nicoletti L, Conte C, Massa S, et al. Congenital malformations potentially affecting respiratory function: multidisciplinary approach and follow-up. Acta Biomed. 2020; 92(1): e2021069. doi:10.23750/abm.v92i1.10591. |
| [16] | Huang JX, Chen Q, Hong SM, Hong JJ, Cao H. Single-direction thoracoscopic lobectomy for children with congenital lung malformation: initial experience. J Cardiothorac Surg. 2023; 18(1): 163. doi:10.1186/s13019-023-02192-7. |
| [17] | Ganarin A, Sgrò A, Garcia Magne M, Volpe A, et al. Thoracoscopy versus thoracotomy for congenital lung malformations treatment: a single center experience. Pediatr Pulmonol. 2021; 56(1): 196-202. doi:10.1002/ppul.25138. |




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML




