-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2025; 15(4): 1006-1012
doi:10.5923/j.ajmms.20251504.33
Received: Mar. 9, 2025; Accepted: Mar. 25, 2025; Published: Apr. 10, 2025

The Prompt Identification and Management of Nephropathies Seen in Post-COVID Syndrome
Safarova Gulnoz A.1, Gadaev Abdugaffor G.2, Ismatova Mekhriniso N.1, Bakhronov Bekhruz B.1
1Bukhara State Medical Institute named after Abu Ali ibn Sino, Bukhara, Uzbekistan
2Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: Safarova Gulnoz A., Bukhara State Medical Institute named after Abu Ali ibn Sino, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The outcomes of antioxidant therapy and dynamic observation in individuals with COVID-19 who did not have any comorbid conditions are presented in the article. Interleukin-6, collagen type IV, and nephrinuria levels in the urine significantly decreased in individuals with mild, moderate, and severe forms of COVID-19 and compromised renal function following six months of monitoring and antioxidant therapy.
Keywords: COVID-19, Interleukin-6, Cystatin-C, Creatinine, Collagen type IV, Nephrinuria, Glomerular filtration rate
Cite this paper: Safarova Gulnoz A., Gadaev Abdugaffor G., Ismatova Mekhriniso N., Bakhronov Bekhruz B., The Prompt Identification and Management of Nephropathies Seen in Post-COVID Syndrome, American Journal of Medicine and Medical Sciences, Vol. 15 No. 4, 2025, pp. 1006-1012. doi: 10.5923/j.ajmms.20251504.33.
1. Introduction
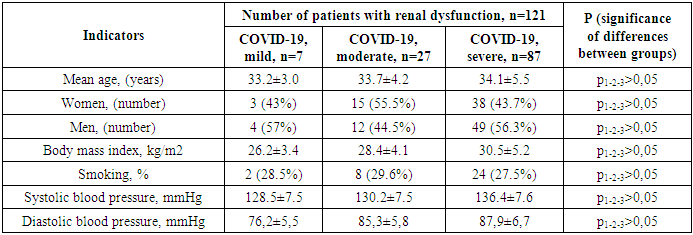
- The COVID-19 infection that shook the world in 2019 poses a great threat to humanity. According to the latest official data, as of November 2022, 244,510 people have been infected in Uzbekistan, of whom 1,637 have died. According to Johns Hopkins University in America, these figures are 630,920,425 and 6,539,051 people worldwide, respectively. The World Health Organization reported that as of March 3, 2023, there were 758 million confirmed cases of COVID-19 worldwide [20]. Although most patients recover and return to their original condition, some have ongoing health problems, a process called post-acute COVID or long COVID. The World Health Organization defines long-term COVID as the persistence of symptoms for three months or more after the initial infection, as well as the appearance of new symptoms in the absence of other causes. The opinion of experts of the said authoritative organization is supported by a number of other observations [19,6].Sometimes, even after a mild infection, persistent symptoms can be observed [2]. Such symptoms include fatigue, shortness of breath, chest tightness, cough, arthralgia, headache, and cognitive dysfunction. According to observers, a protracted course of COVID-19 is observed in 10-30% of patients and lasts more than a year [16,3] However, to date, no studies have been conducted to predict the mechanisms of the development of a long-term course of COVID.Although the main target of the coronavirus is the respiratory system, as the virus spreads through the bloodstream, other organs can also be affected. Because it contains several transmembrane glycoproteins that create conditions for interaction with the cells of the human body. Thus, to date, numerous observations have proven that COVID-19 is a systemic disease that affects not only the respiratory system, but also other systems and causes severe complications [22,21].Its extrapulmonary side effects and complications include disseminated thrombosis, myocardial dysfunction, various arrhythmias, acute coronary syndrome, acute kidney injury, various degrees of gastrointestinal changes, hypoglycemia, ketoacidosis, neurotoxic and neurological complications including cerebral ischemic changes, as well as various ocular and cutaneous reactions. It is known that angiotensin-converting enzyme II (ACE II) receptors serve as a gateway for the virus to enter cells. Since these receptors are present in all organs, the virus can spread through the blood, and its toxic effects can damage tissues and various organs. This, in turn, indicates that the disease becomes systemic. Of course, the direct action of the virus is primarily found in the epithelium of the respiratory tract, which is considered a target organ, and above all in the lungs, where type I cells involved in gas exchange and type II cells producing surfactant are located. Although the virus is found in type II cells of the lung parenchyma, in most cases it remains asymptomatic for 2-7 days after entering the body, after which it begins to spread rapidly throughout the body. Patients develop severe pneumonia and acute respiratory distress syndrome. Then, adjacent organs are damaged, in particular the gastrointestinal tract, renal tubules and liver capillaries, and a release of cytokines is observed. The latent course of the early stages of the disease is due to the fact that the virus hides in a number of cells in the body, creating reservoirs for latent replication. The squamous epithelium of the salivary glands and bronchi is a hidden incubator of viruses. A large number of viruses are released from them, which after a certain time cause viremia and serious changes in the body. Histopathological examination of COVID-19 shows that it has tropism for the epithelium of the kidneys, myocardium, trachea and gastrointestinal tract [10,5,12].Following infection, a vicious cycle of endotheliitis that enhances thrombotic inflammation is maintained by increased ACE II levels on the surface of epithelial cells [1]. This is believed to signal the restoration of control over the renin-angiotensin-aldosterone system (RAAS), thereby triggering cardio- and visceroprotective mechanisms [9]. Of course, the rate and severity of COVID-19 progression are also partly related to the individual condition of each organism and the activation of inflammatory and immune-active cytokines, in particular interleukin (IL)-1β, IL-2, IL-6, IL-7, IL-8, tumor necrosis factor α (TNF-α), and others [4,11]. Patients with hyperactivity of cytokine genes have increased cytokine production. This cytokine storm leads to self-sustaining inflammatory processes, which in turn can lead to widespread endovascular thrombosis and, in extreme cases, to multiple organ failure [15]. The virus disrupts the regulation of RAAS by attaching to ACE II receptors. ACE II receptors are known to be present in large quantities in the epithelium of the heart and kidneys. COVID-19 negatively affects their functioning and leads to significant changes [17]. In addition, changes in RAAS under the influence of the virus cause a disruption of all areas of its control, including renal function, which is manifested by a number of clinical symptoms [8,13]. This is due to excessive activation of the kallikrein-kinin system, in particular bradykinin, in an inflammatory environment, which leads to the development of pulmonary edema. As a result of the disruption of the cardioprotective effect of this system under the influence of the virus, patients develop hypokalemia, decreased blood pressure, arrhythmia, which in turn leads to complications of cardiovascular and renal diseases [18]. The above conditions are detected in 20-30% of patients hospitalized due to the virus, and in people with existing heart and kidney problems, this figure reaches 50%. Although the pathophysiology of cardiovascular diseases, like other pathological processes, is multifactorial, it is associated with increased expression of ACE II receptors on cardiomyocytes and fibroblasts of cardiac tissue, as noted above. This is confirmed by the fact that the virus directly affects endothelial and smooth muscle cells, as well as the heart. The development of myocarditis is due to the viral load and inflammation or activation of immune system cells in the coronary arteries and myocardium due to infection. In addition, as the disease progresses, myocardial ischemia increases, and patients with comorbidities may develop myocardial infarction if such treatments are not used [14,7].The above confirms that COVID-19 is a systemic disease that affects all organs. However, to date, no studies have been conducted in Uzbekistan to determine which internal organs, other than the lungs, are most often affected by this infection in relatively healthy people.From this point of view, the study of kidney damage in patients with COVID-19 is also of great scientific and practical importance.Purpose of the study: To improve the comprehensive assessment and treatment of renal dysfunction in patients with COVID-19 without comorbidities.Materials and methods of the study: The source of the study was 121 patients who were treated for COVID-19 in the Bukhara Regional Infectious Disease Center and the Bukhara Regional Multidisciplinary Medical Center and were under subsequent observation, in whom micro- and macroalbuminuria (above 30 mg / l) was detected in daily urine, without concomitant diseases. Their average age was 33.1 ± 0.8 years, among them there were 65 men and 56 women. Of the study participants, 7 had a mild form of COVID-19, 27 had a moderate form, and 87 had a severe form.Patients included in the study were under continuous observation for six months. Laboratory and instrumental studies were carried out during the first week of the study and after six months. Table 1 below presents a description of the patients included in the study.
|
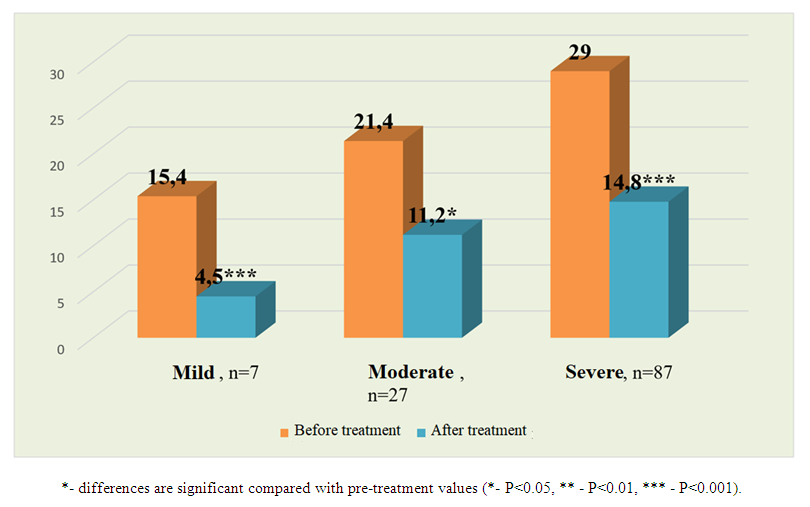 | Figure 1. Changes in interleukin-6 levels (pg/ml) before and after treatment in patients with COVID-19 and varying degrees of renal impairment without comorbidities |
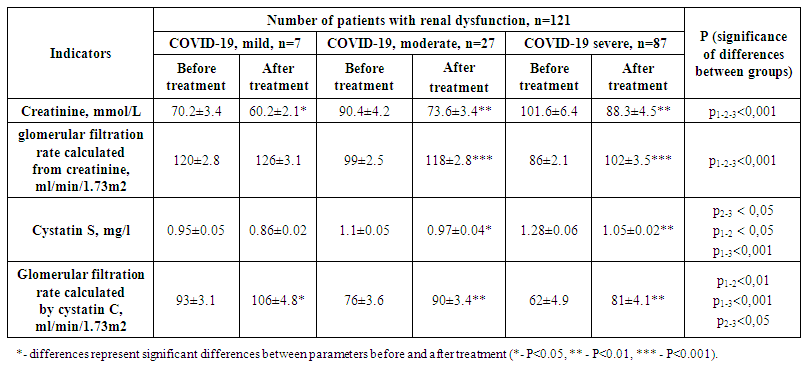 | Table 2. Indices of renal impairment before and after treatment in patients included in the study |
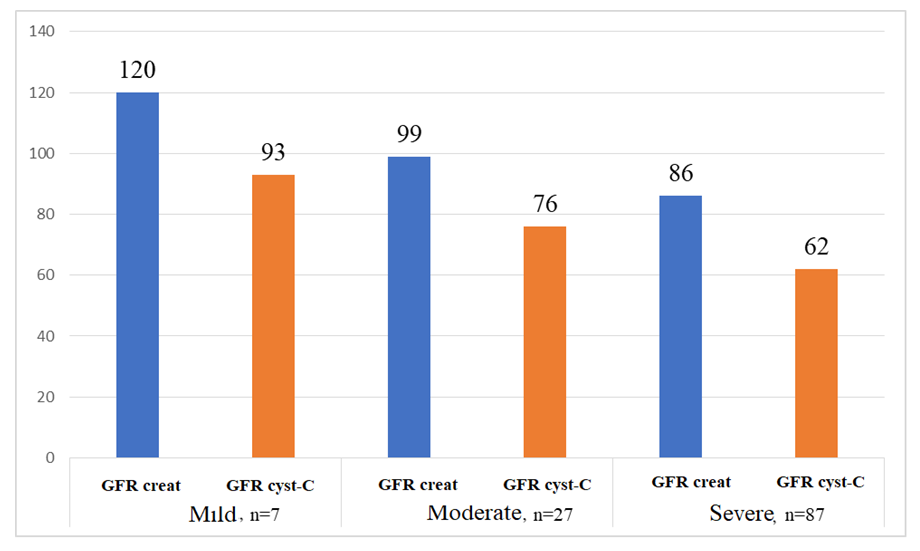 | Figure 2. The estimated glomerular filtration rate (GFR) per minute per 1.73 m2 of body surface area in patients included in the study was determined using creatinine and cystatin C (mL) |
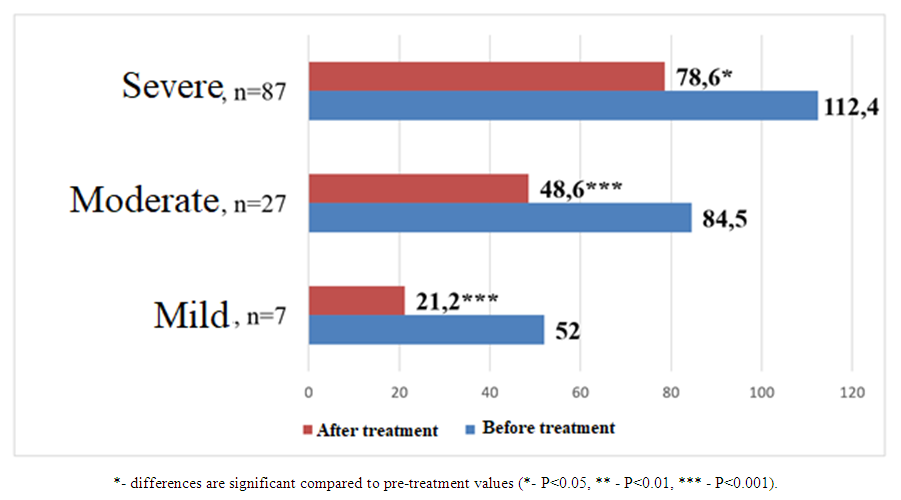 | Figure 3. Comparative analysis of collagen type IV levels (ng/ml) before and after treatment in patients with COVID-19 and varying degrees of renal impairment without concomitant diseases |
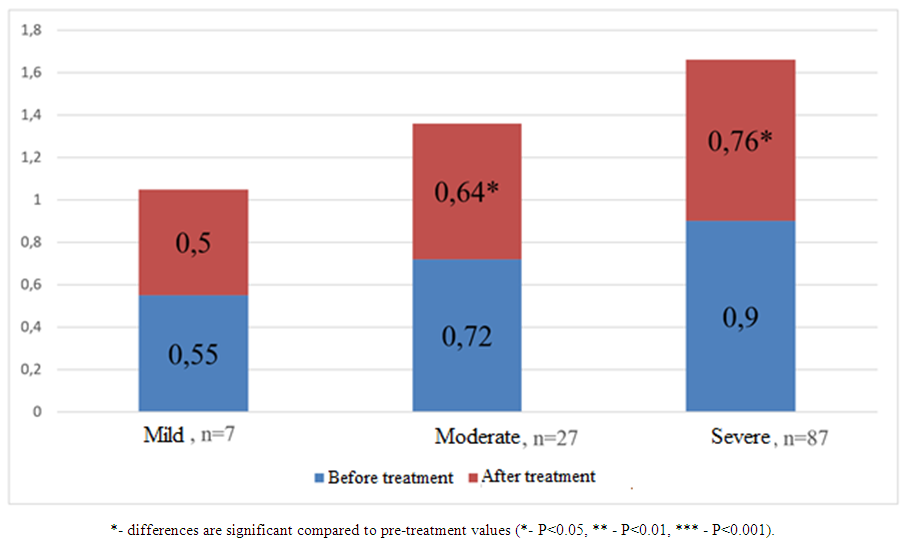 | Figure 4. Comparative analysis of nephrinuria values before and after treatment (ng/ml) in patients with COVID-19 and varying degrees of renal impairment without comorbidities |
2. Conclusions
- After six months of observation and antioxidant therapy, patients with mild, moderate and severe COVID-19 and renal impairment showed significant reductions in urinary interleukin-6, type IV collagen and nephrinuria.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML