Jurayev Jamol Sirojiddinovich
Assistant, Termez branch of Tashkent Medical Academy, Termez, Uzbekistan
Correspondence to: Jurayev Jamol Sirojiddinovich, Assistant, Termez branch of Tashkent Medical Academy, Termez, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The treatment of viral hepatitis C has seen significant advancements with the introduction of modern antiviral drugs, which have demonstrated high efficacy in both clinical and laboratory settings. These drugs, often referred to as direct-acting antivirals (DAAs), have revolutionized the management of hepatitis C by offering cure rates exceeding 95% in many cases. However, despite these remarkable achievements, a subset of patients with chronic viral hepatitis C may experience a relapse following the completion of antiviral therapy. This relapse, characterized by the reappearance of the hepatitis C virus (HCV) in the bloodstream after an initial period of undetectable viral load, poses a significant clinical challenge. The timing of relapse is also an important consideration. Most relapses occur within the first 3 to 12 months (12 to 48 weeks) after the completion of antiviral therapy. This window is critical for monitoring patients through regular follow-up visits and viral load testing to detect any signs of viral recurrence promptly. Early detection of relapse allows for timely intervention and the formulation of a re-treatment strategy tailored to the individual patient's circumstances. In conclusion, while modern antiviral drugs have significantly improved the outcomes for patients with hepatitis C, relapse remains a challenging clinical issue that demands a comprehensive and individualized approach. By understanding the underlying causes of relapse and employing targeted re-treatment strategies, clinicians can optimize the chances of achieving a cure and improving the long-term health outcomes for patients with chronic hepatitis C.
Keywords:
HCV, Relapses, Interferons, Immunocorrector, T-lymphocytes, Extrahepatic markers, InIn
Cite this paper: Jurayev Jamol Sirojiddinovich, Clinical, Immunological and Laboratory Efficiency of Immunocorrector Inin in Patients with Recurrent Chronic Viral Hepatitis C, American Journal of Medicine and Medical Sciences, Vol. 15 No. 3, 2025, pp. 788-792. doi: 10.5923/j.ajmms.20251503.62.
1. Introduction
From the point of view of modern knowledge, recurrence of the virus after treatment of hepatitis C is a complex clinical problem that requires a comprehensive analysis, search for the causes of HCV recurrence and decision-making on repeated treatment of hepatitis. Most often, recurrence of HCV infection occurs during the first 3-12 months (12-48 weeks) after completion of the course of antiviral therapy [1,2,3]. Hepatitis C virus is the main cause of liver diseases. According to the World Health Organization, more than 50 million people are currently infected with this disease. Every year, 350,000 people die from this disease. In 95% of cases, the chronic form develops. The main complications of viral hepatitis C are liver fibrosis, liver cirrhosis and hepatocellular carcinoma [2,4,6,7]. We know that currently viral hepatitis C is treated with modern antiviral drugs. The effectiveness of these drugs has been demonstrated in clinical and laboratory studies. In some cases, patients with chronic viral hepatitis may experience a relapse despite treatment with antiviral drugs. There are many reasons for relapse, and it is certainly an unpleasant event for both the patient and the treating physician [5,8,10].
2. Purpose of the Research
Study of the main immunocorrective (induction of production of the main interferons) properties of the drug DOSTIM™ (InIn) on immunocompetent cells of patients with recurrent chronic viral hepatitis C. To analyze the causes of relapse in patients with relapse of viral hepatitis C, to study the schemes of prevention of relapses and repeated treatment, and to demonstrate their effectiveness.
3. Materials and Methods
To achieve the aim of the study, peripheral blood of patients with chronic viral hepatitis C (CHVH) with formed components aged 29 to 42 years was taken. The material was peripheral whole venous blood of patients with CHVH, which was diluted 5 times with medium 199, the drug InIn was also added to the test tubes for further incubation. Then the blood was incubated for 24 hours at a temperature of 37°C. The method is standardized, generally accepted for studies of spontaneous and induced production of the main mediators of the immune system. The samples were carried out in parallel in comparison with biological material from healthy individuals. After that, the supernatant was collected and the content of IFNα, IFNγ and IFN-lambda were assessed by enzyme-linked immunosorbent assay (ELISA) using Vector-Best test systems, Russia, 2024. The study included several stages: Stage 1 – evaluation of the main interferons in the blood plasma (existing interferon production) of patients with chronic hepatitis C; Stage 2 – evaluation of spontaneous interferon production, that is, without incubation with the drug; Stage 3 – induced interferon production, in incubation with the drug.
4. Results and Discussion
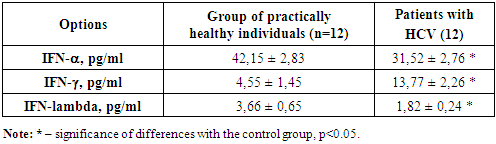
Stage 1 – evaluation of the main interferons in the blood plasma (available interferon production) of patients with chronic hepatitis C. The obtained data are presented in Table 1.Table 1. Study of the main interferons in patients with chronic hepatitis C, (M±m)
 |
| |
|
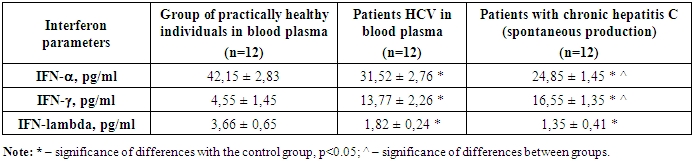
We studied the available values of the main interferons in the blood plasma of patients with CHC. The analysis showed that IFN-alpha in the control group averaged 42.1 ± 2.83 ng/ml. With CHC, the IFN-alpha level was 31.52 ± 2.76 ng/ml. It is evident that in the group of patients with CHC, there is a reliable suppression of the concentration of interferon-alpha compared to the value of the control group. According to numerous literature data, it is known that the content of serum interferon, as well as spontaneous and induced production, is sharply reduced against the background of chronic viral hepatitis, which was also revealed by us. This fact once again proves the low antiviral activity of interferons against the background of the natural course of the chronic viral process. From this we can say that the level of antibodies to IFN-alpha will also be reduced in relation to the control data. Thus, the level of IFN-alpha is reduced by 1.8 times in relation to the control. The analysis showed that the IFN-lambda level in the control group was 3.66 pg/ml, and in the group of patients with chronic hepatitis C - 1.82 pg/ml, which indicates a reliable decrease of almost 2 times from the normal values, most likely this indicates the depletion of antiviral immunity. The analysis showed that IFN-gamma in the control group averaged 4.55 ± 1.45 ng/ml. With chronic hepatitis C, the IFN-gamma level is 13.77 ± 2.26 ng/ml. It is evident that in the group of patients with chronic hepatitis C, there is a reliable suppression of the concentration of Interferon-gamma compared to the value of the control group. Also, according to numerous literature data, it is known that the content of serum interferon can be different. Such activity of interferon-gamma against the background of the natural course of the chronic viral process can be caused by chronic activation of T-cell immunity, in particular, T-lymphocytes. Hence, it can be said that the level of IFN-gamma is increased by 2.4 times compared to the control. This often explains the mechanism of delayed-type hypersensitivity due to the activation of T-cell immunity.Consequently, it was revealed that the content of interferons alpha and gamma in the blood plasma of patients with chronic hepatitis C is due to the activation of T-cell immunity and suppression of natural antiviral immunity.According to modern concepts, the most significant mechanism of death of HCV-infected hepatocytes is apoptosis due to the cellular immune response, especially mediated by cytotoxic T cells (CTL). A number of studies confirm the leading role of the latter not only in the clearance of the virus, but also in the pathogenesis of liver damage in viral hepatitis. Higher levels of IFN-gamma in patients with chronic hepatitis C determine the leading role of these cytokines in the pathogenesis of the systemic inflammatory response in this disease. Thus, the increased content of IFN-gamma, CD8+ T-cytotoxic cells in patients with chronic hepatitis C, obviously explains the significantly more pronounced cytolysis in this category of patients, which allows us to assert that in chronic hepatitis C there is a more pronounced inflammatory-necrotic inflammation in the liver parenchyma, determined by the systemic inflammatory response aimed at eliminating the pathogen. It is known that more pronounced inflammatory-necrotic changes in patients lead to the development of cirrhosis more often, despite the younger age of patients. The next stages of the study are the assessment of spontaneous interferon production, i.e., without incubation with the drug, and stage 3 - induced interferon production, in incubation with the drug in order to identify the positive or negative effect of the drug on the immunocompetent cells of the body to improve the effectiveness of the quality of antiviral therapy.The results of the second stage, i.e., the study of spontaneous interferon induction, are presented in Table 2. We have shown that spontaneous production of the main interferons was detected in the group of patients with chronic hepatitis C. It is shown that without incubation of peripheral blood lymphocytes of patients with chronic hepatitis C with the drug, reliable changes in the production of alpha and gamma interferons in the blood plasma are observed after a day of incubation in a thermostat at a temperature of 37 degrees.Table 2. Study of spontaneous interferon production in patients with chronic hepatitis C (without incubation with the drug), (M±m)
 |
| |
|
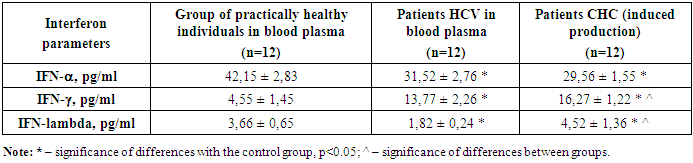
Thus, from the data obtained in the table it is evident that the level of IFN-lambda decreased in spontaneous production, which once again indicates the depletion of the antiviral potential of this interferon. Although the difference was not reliable, the expected result of further decrease in IFN-lambda in the blood is visible.It is evident that the level of IFN-alpha was significantly reduced in the group of patients with chronic hepatitis C with spontaneous production of interferons. It is evident from Table 2 that spontaneous production of IFN-alpha in patients with chronic hepatitis C in relation to the values in blood plasma was reduced by 1.3 times, which indicates a decrease in the production of IFN-alpha.Thus, it is evident that the level of IFN-gamma was significantly increased in the group of patients with chronic hepatitis C with spontaneous production of interferons. It is evident from Table 2 that spontaneous production of IFN-gamma in patients with chronic hepatitis C in relation to the values in blood plasma was reduced by 1.2 times, which indicates an increase in the production of IFN-gamma in spontaneous production, which once again proves the activity of T-cell immunity and the severity of the delayed-type hypersensitivity reaction.Stage 3 of the study was devoted to the study of induced production of interferons alpha and gamma by the effect of the medicinal substance InIn on immunocompetent cells of patients with chronic hepatitis C. The results obtained are presented in Table 3. The goal was to evaluate the possibility of producing the main interferons alpha and gamma in patients with chronic hepatitis C under the influence of the medicinal substance InIn. It is known that with chronic hepatitis C, there is pronounced immunosuppression of immunity due to the viral load on the patient's immunity.Table 3. Study of induced production of interferons in patients with chronic hepatitis C (with incubation with the medicinal substance), (M ± m)
 |
| |
|
As can be seen, the level of IFN-lambda in the induced production increased, which indicates the activation of immune cells that were incubated with the medicinal substance InIn. The most interesting thing is that the reliable increase in IFN-lambda is 2.4 times compared to the data of patients with chronic hepatitis C in the blood plasma.Thus, it is clear from Table 3 that the level of IFN-alpha in the induced production was significantly reduced compared to the control values in the group of patients with chronic hepatitis C. It is clear from Table 3 that induced production was not observed under the influence of the medicinal substance InIn on the lymphocytes of patients. That is, we see that the induction of IFN-alpha is not observed, which indicates that this drug does not activate the production of IFN-alpha, which is an important antiviral mediator. It is clear that IFN-alpha did not increase or decrease, it remained at its values.As for IFN-gamma, here we see an increase in the production of this interferon. Moreover, in patients with chronic hepatitis C, with induced production, an increase in IFN-gamma is observed by 1.2 times higher than in the plasma of patients with chronic hepatitis C. It follows that this drug activates the production of IFN-gamma, which is an important point in increasing immunity, especially humoral interferon immunity, which has pronounced antiviral and anti-inflammatory properties.This picture will be clearer with a comparative description of the production of interferons, that is, with a visual analysis of the results obtained. For this purpose, in Table 4 we present an analysis between the studied blood groups, that is, we studied in the blood plasma of patients with chronic hepatitis C, then spontaneous induction, that is, without incubation with the drug, the so-called internal control on lymphocytes of patients with chronic hepatitis C and induced induction with the drug InIn, in order to assess the presence of interferon production.Table 4. Comparative analysis of interferon production in patients with chronic hepatitis C, (M±m)
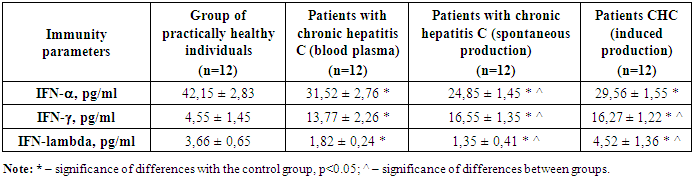 |
| |
|
A comparative analysis of the induced production of the main antiviral interferons was carried out with the values of spontaneous production, which served as an internal control for our studies. As can be seen from Table 4, the values of the main interferons alpha, gamma and lambda are presented. It is evident that IFN-alpha in the blood plasma of patients with chronic hepatitis C was 31.5 pg / ml, while with spontaneous production 24.85 pg / ml, and with induced production - 29.56 pg / ml. It should be noted that normally this indicator is 42.15 pg / ml. Thus, it is evident that in patients there is suppression of IFN-alpha. When incubating immune cells without the drug InIn, a reliable decrease in the already reduced value of IFN-alpha is observed. When incubating with the drug InIn, a reliable increase in IFN-alpha is observed compared to the value of the internal control, that is, with the value of spontaneous production of IFN-alpha. Therefore, we see low values of an important antiviral cytokine such as IFN-alpha in the group of patients with chronic hepatitis C. Moreover, an additional decrease is observed during incubation of immune cells, which indicates that a deep humoral immunodeficiency is visible in patients with chronic hepatitis C. And during incubation with the medicinal substance InIn, an insignificant but reliable increase in IFN-alpha is observed.Further, it is clear from Table 4, the values of the main interferons alpha, gamma and lambda are presented. It is clear that IFN-gamma in the blood plasma of patients with chronic hepatitis C was 13.77 pg / ml, while with spontaneous production 16.55 pg / ml, and with induced production - 16.27 pg / ml. It should be noted that normally this indicator is 4.55 pg / ml. Thus, it is clear that patients have an increase in IFN-gamma. When incubating immune cells without the drug InIn, a reliable increase in the IFN-gamma value is observed compared to the data in the blood plasma. When incubating with the drug InIn, an unreliable difference in IFN-gamma is observed compared to the internal control value, that is, with the value of spontaneous IFN-gamma production, and a reliable increase in IFN-gamma compared to the value in the blood plasma. Therefore, we see an increase in an important antiviral T-cell cytokine such as IFN-gamma in the group of patients with chronic hepatitis C. Moreover, an additional increase in IFN-gamma is observed in spontaneous and induced production by immune cells, which indicates activation of T-cell immunity in patients with chronic hepatitis C, which is manifested in a delayed-type hypersensitivity reaction.Table 4 shows the values of the main interferons alpha, gamma and lambda. It is evident that IFN-lambda in the blood plasma of patients with chronic hepatitis C was 1.82 pg/ml, while with spontaneous production it was 1.35 pg/ml, and with induced production it was 4.52 pg/ml. It should be noted that normally this indicator is 3.66 pg/ml. Thus, it is evident that in patients with chronic hepatitis C there is a reliable suppression of IFN-lambda. When incubating immune cells without the drug InIn, there is a reliable decrease in the IFN-lambda value compared to the control data, but no difference was observed with the value in the blood plasma. When incubating with the drug InIn, there is a reliable increase in the IFN-lambda value compared to the internal control value, that is, with the spontaneous value and in the blood plasma. Therefore, we see an increase in the important antiviral IFN-lambda in the group of patients with chronic hepatitis C against the background of incubation with the medicinal substance InIn, which has a pronounced antiviral immunotropic effect, increasing the activity of immune cells to produce the main antiviral interferons gamma and lambda.
5. Conclusions
IFN-alpha in the blood plasma of patients with chronic hepatitis C was 31.5 pg/ml, while with spontaneous production it was 24.85 pg/ml, and with induced production it was 29.56 pg/ml, and in the control it was 42.15 pg/ml. Patients showed a pronounced suppression of IFN-alpha. Spontaneous production of IFN-alpha without the drug InIn was significantly reduced. Induced production with the drug InIn was significantly increased compared to the value of the internal control, that is, with the value of spontaneous production of IFN-alpha. Consequently, low values of an important antiviral cytokine such as IFN-alpha were revealed in the group of patients with chronic hepatitis C. When incubated with the drug InIn, an insignificant but reliable increase in IFN-alpha was observed. IFN-gamma in the blood plasma of patients with chronic hepatitis C was 13.77 pg/ml, while with spontaneous production it was 16.55 pg/ml, and with induced production it was 16.27 pg/ml, and in the control, it was 4.55 pg/ml. A reliable increase in IFN-gamma was observed in patients. Spontaneous production of IFN-gamma without the drug InIn was significantly increased compared to the data in the blood plasma. Induced production with the drug InIn was unreliable IFN-gamma compared to the internal control value, that is, with the value of spontaneous production of IFN-gamma, and IFN-gamma was significantly increased compared to the value in the blood plasma. Consequently, an increase in IFN-gamma was revealed in the group of patients with chronic hepatitis C. There is an additional increase in IFN-gamma during spontaneous and induced production by immune cells, indicating activation of T-cell immunity in patients with CHBV, which manifests itself in a delayed-type hypersensitivity reaction.IFN-lambda in the blood plasma of patients with CHBV was 1.82 pg / ml, while with spontaneous production it was 1.35 pg / ml, and with induced production - 4.52 pg / ml, and in the control - 3.66 pg / ml. In patients with CHBV, there is a reliable suppression of IFN-lambda. Spontaneous production without the drug InIn significantly decreased IFN-lambda compared to the control data, but no difference was observed with the value in the blood plasma. Induced production with the drug InIn significantly increased IFN-lambda compared to the internal control value, that is, with the spontaneous value and in the blood plasma. Consequently, an increase in the important antiviral IFN-lambda was revealed in the group of patients with chronic hepatitis C against the background of incubation with the medicinal substance InIn, which has a pronounced antiviral immunotropic effect, increasing the activity of immune cells to produce the main antiviral interferons.
References
| [1] | Jurkin A.T. Viral hepatitis. Textbook. - St. Petersburg, 2004, 62 p. |
| [2] | Roncoleukin immunotherapy of chronic viral hepatitis C. Method, rec. comp.: Sklyar L.F., Ivanis V.A., Markelova E.V. // Vladivostok, 2003-36 p. |
| [3] | Lobzin Yu.V. Handbook of infectious diseases. - St. Petersburg: Foliant Publishing House, 2000. - 936 p. |
| [4] | Mitsura V.M., Zhavoronok S.V., Krasavtsev E.L. Use of Roncoleukin in complex therapy of chronic hepatitis C. Method, rec. // Gomel, 2004. 34 p. |
| [5] | Radchenko V.G. et al. Fundamentals of Clinical Hepatology. Liver and Biliary System Diseases. - SPb.: Dialect Publishing House; M.: 2005. - 864 p. |
| [6] | RF Patent No. 2265447 dated 19.05.2004. Method for the Treatment of Chronic Viral Hepatitis. |
| [7] | Podymova S.D. Liver Diseases. - M.: Meditsina, 1998, 704 p. |
| [8] | Popovich A.N., Egorova V.N. Interleukin 2: Clinical Application Experience. (Second Edition) - SPb.: Publishing House “Izdatelsky Dom Novosti Pravoporyadka”, 2006. - 36 p. |
| [9] | Mayer K.P. Hepatitis and Its Consequences. - M., 2002. - Ch. 4.2. – P. 78-110. |
| [10] | Ignatova T.M. Chronic hepatitis C: clinical and morphological characteristics, course, treatment: Diss. … Doctor of Medicine. - M., 2000. - 235 p. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


