-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(5): 1229-1237
doi:10.5923/j.ajmms.20241405.19
Received: Mar. 31, 2024; Accepted: Apr. 29, 2024; Published: May 11, 2024

Identifying Key Factors Impacting Hospitalization Outcomes in Variceal Upper Gastrointestinal Bleeding: A Machine Learning Approach
Ismati Amir Olimovich1, Mamarajabov Sobirjon Ergashevich1, Anosov Victor Davidovich2
1Samarkand State Medical University, Samarkand, Republic of Uzbekistan
2City Clinical Hospital No. 15 named after O.M. Filatov, Moscow, Russian Federation
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Managing patients with variceal bleeding from upper gastrointestinal (GI) tract is always a challenging task not only due to the various causes leading to this condition, but also because of the multitude of factors capable of impacting hospitalization outcomes. Objective: The aim of this study was to investigate the degree of influence of factors on 30-day hospitalization outcomes using machine learning (ML) methods. Methods: A retrospective dataset was collected from 105 patients and included clinical, anamnestic, laboratory, instrumental data. Subsequently, the entire database was divided into two samples with differing levels of data completeness. The obtained samples were processed using ML tools in two stages: imputation with usage model of multiple imputation by chained equations (MICE), and factor importance analysis using tuned random forest models. The primary outcome was death or successful discharge. Results: There were not only well-known predictors of mortality found among the most prognostically valuable indicators, but also factors that hold promise for the role of predictor in scientific community. The top-10 most prognostically significant factors were found to be: ferritin, blood urea level, arterial pressure, procalcitonin, creatinine, lactate, amylase, activated partial thromboplastin time (APTT), white blood cell count, aspartate aminotransferase (AST). Conclusion: Usage of advanced methods confirmed the significance of already known and validated predictors of mortality, contributed not only to the development of newly proposed predictors by scientific community in recent times, but also to those yet unexplored.
Keywords: Variceal bleeding, Prognostically important, Outcome, Mortality, Predictor
Cite this paper: Ismati Amir Olimovich, Mamarajabov Sobirjon Ergashevich, Anosov Victor Davidovich, Identifying Key Factors Impacting Hospitalization Outcomes in Variceal Upper Gastrointestinal Bleeding: A Machine Learning Approach, American Journal of Medicine and Medical Sciences, Vol. 14 No. 5, 2024, pp. 1229-1237. doi: 10.5923/j.ajmms.20241405.19.
Article Outline
1. Introduction
- Upper gastrointestinal bleeding (UGIB) is a multifactorial acute pathology, still representing one of the common reasons of emergency admissions [1,2]. The highest number of fatal cases among patients with bleedings from upper gastrointestinal (GI) tract is traditionally encountered in group with variceal hemorrhage [3,4]. Mortality associated with this condition depends on a multitude of factors and increases with age and number of comorbidities [5]. In addition, there are specific factors that assist physicians in stratifying patients into risk groups. The most reliable of these factors are often mentioned as predictors in scientific literature [6].There is a plethora of well-known predictors of mortality, frequently referenced in clinical guidelines [7,8,9]. The most effective predictors in terms of prognosis are already incorporated into simple scoring systems for predicting clinical outcomes [10,11,12,13,14,15].Amidst the rapid advancement of artificial intelligence (AI) technologies, machine learning (ML) methods have become commonplace in the physician's toolkit. Over the past several years, there has been a growing body of researches utilizing ML techniques [16,17]. This fact indicates that scientists are increasingly favoring advanced statistical analysis methods. Implementation of predictive models emerges the possibility of obtaining valuable insights into the factors influencing clinical outcomes based on trained data.Despite the existing number of well-known predictors of mortality, there remains a pertinent need for new researches aimed at potential identifying new predictors and re-evaluating the significance of already known ones. In this study, we focus our attention on analyzing a broad spectrum of patient data using advanced analytical methods based on ML. The aims of this research were to investigate factors in group of patients with variceal UGIB, identifying predictors of 30-day mortality among them, as well as to reassess already known predictors of 30-day mortality in order to use in clinical practice with high efficiency and assist physicians in more precise risk stratification with subsequent reducing mortality.
2. Materials and Methods
2.1. Study Design
- A single-center retrospective study was conducted at City Clinical Hospital No. 15, named after O.M. Filatov, affiliated with the Department of Healthcare of the City of Moscow, Russian Federation. The study database included patients with UGIB within a timeframe from 2020 to 2023.The inclusion criteria for patients in the study were as follows: age ≥18 years, diagnosed variceal UGIB. The exclusion criteria were as follows: absence of performed endoscopic examination, refusal to sign provided informed consent for inclusion into the study, discharge from the hospital at the patient’s request.
2.2. Data Collection
- The study involved 105 patients with variceal UGIB. Patient information was gathered from the specialized electronic medical record system for the period from 2020 to 2023. All parameters in the database included 213 factors: anamnestic, clinical, laboratory, endoscopic, and some instrumental data.Anamnestic data included information about: patient's gender, age, height, weight, known harmful habits, list of comorbidities, some heart procedures, outpatient medication use, initial manifestations of GIB and the duration since their onset, site of detection (pre-hospital or during hospitalization period), possible previous episodes of UGIB according to medical documentation or patient's report.Laboratory data was collected on the first, third, and fifth days from the moment of confirmation of UGIB and included parameters from complete blood count (CBC), biochemical analysis (kidney and liver function tests, albumin, total protein, glucose, amylase), ferritin, lactate dehydrogenase (LDH), C-reactive protein (CRP), procalcitonin, D-dimer, venous or arterial blood pH, electrolyte levels (potassium, sodium, chloride), lactate level, arterial blood gas levels, coagulation profile (international normalised ratio, activated partial thromboplastin time, fibrinogen, prothrombin time).Endoscopic data included information not only about the priority source of UGIB (variceal), but also about secondary less life-threatening sources (ulcers, erosions, neoplasms, angiodysplasias) in case of their presence: quantity, localization, depth of defect, dimensions when possible to measure, Forrest classification for ulcer bleedings, methods of performed endoscopic hemostasis (injection, electrocoagulation, argon plasma coagulation, clipping, band ligation), as well as calculation of the area of major bleeding defects.Instrumental data included: results of computed tomography (CT) of lung parenchyma, echocardiography with determination of left ventricular ejection fraction (LVEF).Clinical data included information on patient's condition during the first, third, and fifth days from the instrumental confirmation of UGIB, specifically: heart rate and rhythm, systolic and diastolic blood pressure, information about vasopressor support of hemodynamics, patient's level of consciousness, body temperature, information on 24-hour urine volume, presence of abdominal pain with clarification of its localization, respiratory rate during spontaneous breathing or mechanical ventilation of lungs. Additionally, the medications taken by the patient were recorded and classified into groups: anticoagulants, antiplatelet agents (including acetylsalicylic acid), proton pump inhibitors (PPI), antihypertensive drugs, antibiotics, analgesics, corticosteroids, non-steroidal anti-inflammatory drugs (excluding acetylsalicylic acid) (NSAID), biological therapy (tocilizumab, levilimab, olokizumab) in patients with confirmed COVID-19. Information on gastroprotective therapy and conservative hemostatic measures (hemostatic agents, Sengstaken–Blakemore tube) after the detection of UGIB was also registered. Hemotransfusion therapy (red blood cell, platelet and plasma transfusions) was recorded in milliliters. Clinical data also included information on recurrence of bleeding, information about surgery performed in case of failure of endoscopic and endovascular hemostasis and possible complications after surgical intervention, length of hospital stay (LOS), some indices and scoring systems calculated basing on collected information: body mass index, Charlson Comorbidity Index (CCI) in scores and percentage expression, average arterial pressure based on measured systolic and diastolic values of blood pressure, categorization of minimally registered level of consciousness during the first, third, and fifth days into three severity groups, namely 0-9 points according to the Glasgow Coma Scale (GCS), 10-12 points, 13-15 points, calculation of shock index, calculation of ASA score, total number of comorbidities. Clinical outcomes were formed in a binary format: survival and mortality.
2.3. Statistical Analysis
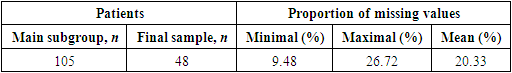
- One of the weaknesses of this study lies in the fact that filling in over 200 clinical parameters for each patient appears challenging, particularly in the setting of emergency hospital. Due to this issue, associated with frequent inability to fill all factors in the database, a decision was made to resort to selecting a portion of patients from the most complete section of the database, followed by imputation of missing values using advanced analytical algorithms based on ML.At the first stage of statistical analysis 48 patients with variceal bleeding (mean proportion of missing values - 20.33%) out of 105. Selected group was identified as final sample (Table 1).
|
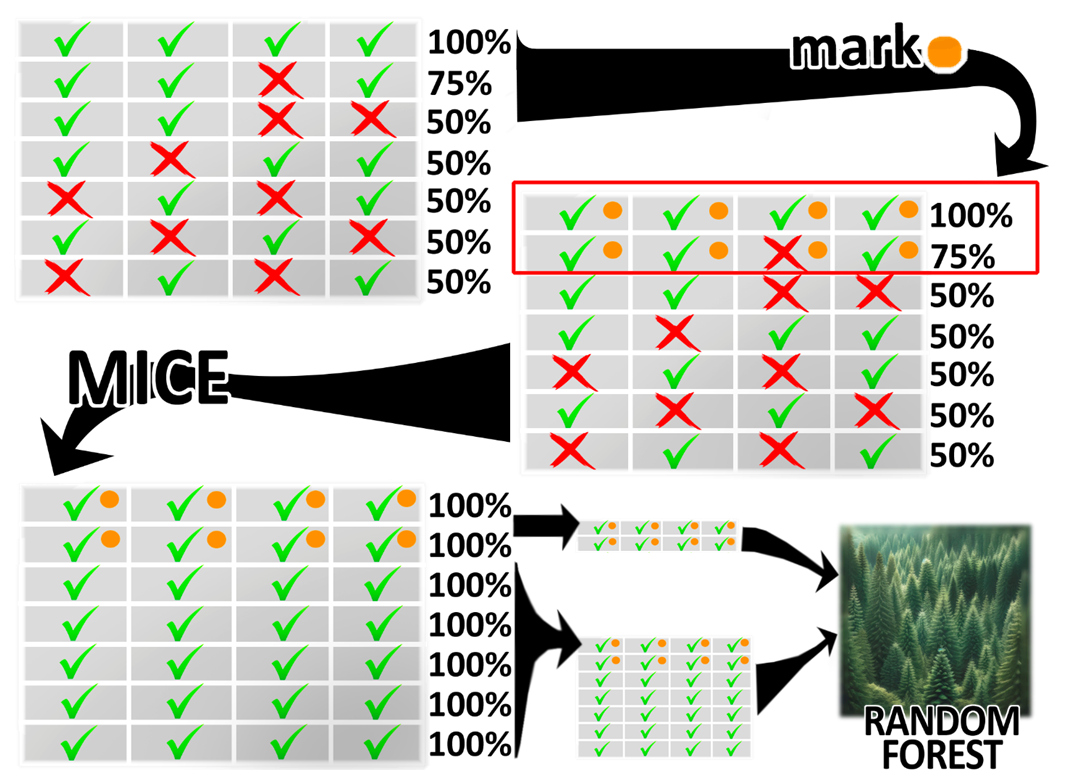 | Figure 1. Schematic overview of implemented statistical analysis |
|
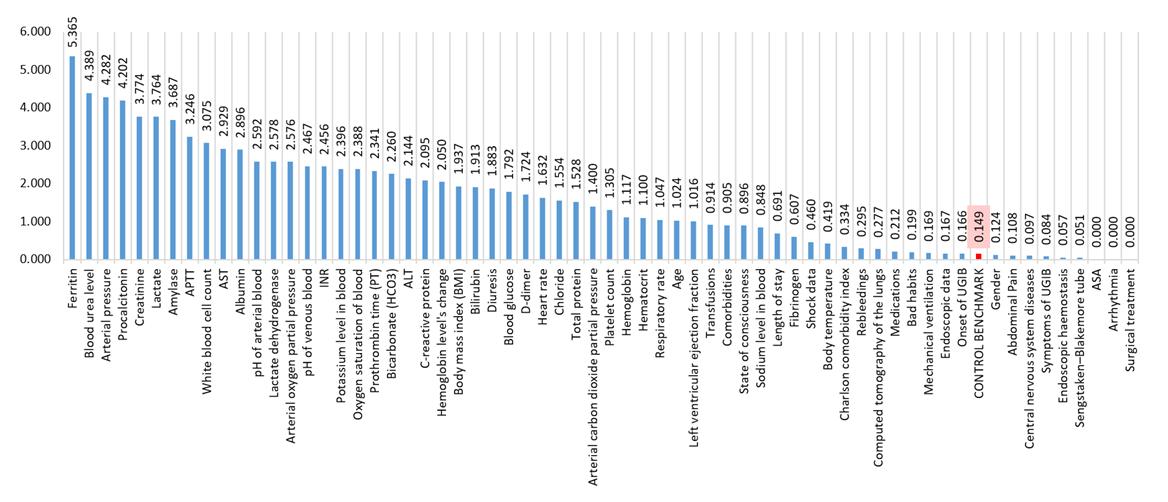 | Figure 2. Significance of factors according to random forest analysis of factors associated with variceal bleedings |
3. Results
3.1. Study Population
- There were more women registered in group than men. One in every six patients had a documented history of UGIB. Liver cirrhosis was present in 81% of the group, diabetes mellitus and CHF were encountered in one-fifth of the patients, hypertension was observed in 47% of the patients, and one-third of the group had alcohol abuse issues.BMI, body mass index; UGIB, upper gastrointestinal bleeding; PUD, peptic ulcer disease; MI, myocardial infarction; HF, heart failure; NSAID, non-steroidal anti-inflammatory drugs; CRP, C-reactive protein; INR, international normalised ratio; ASA, American Society of Anesthesiologists physical status classification system; LOS, length of stay.The hemoglobin level often fell within the range of moderate anemia, while renal indicators were frequently elevated. Up to three-quarters of the group required various forms of blood transfusion therapy. Initial manifestations of UGIB were most commonly observed in the form of hematemesis, rebleeding rates reached up to 8%, and mortality was at 41%.
|
3.2. Predictors of Outcome
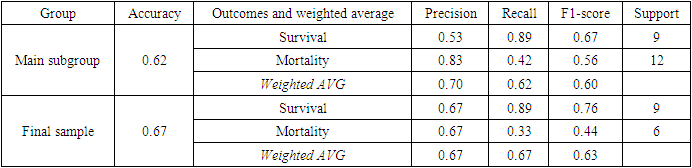
- All the metrics obtained during validation stage on test groups, representing 20% of main subgroup and 30% of final sample, indicate that predictive model is to some extent more proficient in identifying factors influencing survival rather than mortality, likely due to the overwhelming majority of surviving patients in all groups, whose data were absorbed by random forest predictive model during its training phase (Table 4). Recall for mortality was low, however, recall for survival in both main subgroup and final sample, as well as precision for mortality in main subgroup showed high results.
|
4. Discussion
- A comprehensive approach based on usage of ML tools was utilized within this study. Patient database containing all relevant information was preprocessed using MICE algorithms and subsequently subjected to processing using random forest models (Figure 1). It is assumed that predictors exceeding the control benchmark parameter in histogram has greater prognostic influence, than those which are below control parameter. However, factors with relatively low significance values are not useless but require further analysis to identify conditions allowing predictors to reliably correlate with hospitalization outcomes.The majority of results regarding the significance of factors did not raise doubts, as they logically fit into the traditional clinical picture of the pathological condition. However, there were outliers, such as the peak indicator of ferritin’s significance in patients with variceal bleeding, number of researches on which is still insufficient [18,19]. It appears that depletion of iron reserves may serve as a rather reliable predictor of mortality among patients with variceal UGIB (Figure 2).Urea level of blood and creatinine are renowned for their predictive significance and have been utilized in prognostic scoring systems for quite some time: the latest notable study was the development of ABC scoring system, which includes creatinine levels in the algorithm for calculating mortality prognosis [13,15]. The analysis conducted in this study also confirms high efficacy of these predictors in forecasting clinical outcomes.Arterial pressure, identified in this research as one of the strongest influencing factors on clinical outcomes, has long been a reliable predictor and is still utilized in well-known prognostic scoring systems used in cases of UGIB such as the Rockall Score (RS), Glasgow-Blatchford Score (GBS), AIMS65, Cedars-Sinai Medical Center Predictive Index (CSMCPI), Progetto Nazionale Emorragia Digestive Score (PNED) [10,11,12,13,14].Procalcitonin has previously already been discussed among scientists in context of increased frequency of variceal bleedings among patients with cirrhosis [20], and studies have emerged associating higher levels of procalcitonin to increased frequency of GIB [21]. Sepsis is a pathological condition with high mortality, and this study can join to research pool confirming the prognostic value of procalcitonin in patients with variceal UGIB: procalcitonin’s significance was among especially noticeable ones in the results.According to the correlation potential of LDH with clinical outcomes in patients with UGIB, compelling scientific literature has not yet been accumulated. However, in this study, unexpected elevated levels were found not only for LDH but also for participant of the chemical reaction in which LDH takes part. This reaction participant is called lactate. Blood lactate has been noted in scientific literature not only as a useful predictor in patients with UGIB but also as a cost-effective option in clinical practice applicable in forecasting various scenarios: mortality, recurrent bleeding, the need for transfer to the intensive care unit, and the need for transfusion therapy [6,22,23].The level of white blood cells has previously been repeatedly noted as an independent predictor of mortality in patients with UGIB [24,25]. In this study, we also confirmed the high significance of this predictor in managing patients with UGIB of variceal etiology.Factors such as blood amylase, APTT and AST, according to the obtained results, were also among ten the most important prognostic indicators. They have been noted in studies defining their significance in predicting the occurrence of variceal UGIB in at-risk patient groups [26,27]. However, they were not identified in compelling scientific researches determining the value of these predictors in predicting 30-day mortality.Until recently, the ability to predict clinical outcomes in patients with UGIB has been limited to a small number of factors upon which subsequent treatment strategies were structured. However, the rapid proliferation of AI tools has transformed the exploitation of its capabilities into commonplace practice. ML, which is a branch of AI, enables the utilization of advanced analytical methods and the extraction of valuable datasets as output. The analysis conducted in this scientific work, utilizing various ML models, not only allows for a reassessment of acknowledged predictors but also enables a deeper exploration of previously unknown factors requiring validation.However, our study has several limitations. Firstly, the work is single-center and retrospective. Secondly, patient data collection was conducted at an emergency center, which made it difficult to completely fill out more than 200 parameters for each patient, leading to the adoption of MICE method. Thirdly, small patient samples with an unbalanced ratio in terms of target indicator (hospitalization outcome: survival or mortality) were utilized, which could to some extent affect the inability of random forest predictive model to adequately perform testing phase of analysis on patients with lethal outcomes, potentially leading to the lack of confident manifestation of certain predictors of lethality in results of final samples. Thus, some factors that appeared insignificant in this study may demonstrate statistical significance in larger scientific works. Fourthly, the random forest method is highly accurate but complex to interpret regarding its analysis results, necessitating further research into the identified predictors. Lastly, the ML methods used are among the most reliable modern imputation and statistical analysis techniques, however, achieving the highest accuracy requires substantial computational power—supercomputers—which is currently a tool difficult to access.
5. Conclusions
- Through ML not only a revision of already recommended predictors was performed, but also the identification of factors with high prognostic significance, previously overlooked. It was revealed based on advanced analysis methods utilizing ML that top-10 the most important factors were: ferritin, blood urea level, arterial blood pressure, procalcitonin, creatinine, lactate, amylase, APTT, white blood cell count, AST. However, conducted research may be insufficient for practical application of identified predictors in stratifying patients due to limitations of research. Therefore, we recommend multicenter studies with a larger number of participating patients to effectively identify those at high risk of adverse clinical outcomes.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML