-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(5): 1238-1241
doi:10.5923/j.ajmms.20241405.20
Received: Apr. 5, 2024; Accepted: Apr. 28, 2024; Published: May 11, 2024

Ability of Erythrocytes to Absorb Different Endogenous Substances
Saidov Alonur Bakhtinurovich1, Layla Jumaboevna Gurbanova2
1Professor, Head of the Department of Hematology, Transfusiology and Clinical Laboratory Tashkent Medical Academy, Forobiy 2, Tashkent, Uzbekistan
2Researcher, Department of Hematology, Tashkent Medical Academy, Forobiy 2, Tashkent, Uzbekistan
Correspondence to: Layla Jumaboevna Gurbanova, Researcher, Department of Hematology, Tashkent Medical Academy, Forobiy 2, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In order to study the sorption capacity of erythrocytes in erythrocyte masses prepared in different forms, erythrocyte mass was isolated from the blood of 48 male donors of Uzbek nationality aged 21-62 at the Republican Blood Transfusion Center. leukofiltered. The ability of erythrocytes to absorb endogenous substances such as total protein, albumin, glucose, cholesterol, triglyceride, high-density lipoprotein (HDLP), low-density lipoprotein (LDLP), urea and creatinine was studied. In the obtained results, there are significant changes in the ability of sorption of various endogenous substances in washed erythrocytes. In this case, the rate of sorption of endogenous substances mainly increases. In irradiated erythrocytes, there are significant changes in the ability to absorb various endogenous substances. In this case, not only sorption of endogenous substances, but also reverse processes, i.e. desorption, are observed. In frozen erythrocytes, there are significant changes in the ability to absorb various endogenous substances. In this case, the ability to sorb endogenous substances is observed for all substances, and for triglycerides, the reverse process, desorption, is also observed. In leukofiltered erythrocytes, there are significant changes in the ability to absorb various endogenous substances. In this case, erythrocytes almost lose their ability to absorb many endogenous substances.
Keywords: Donor, Blood, Erythrocyte, Sorption, Desorption
Cite this paper: Saidov Alonur Bakhtinurovich, Layla Jumaboevna Gurbanova, Ability of Erythrocytes to Absorb Different Endogenous Substances, American Journal of Medicine and Medical Sciences, Vol. 14 No. 5, 2024, pp. 1238-1241. doi: 10.5923/j.ajmms.20241405.20.
Article Outline
1. Introduction
- The predominance of the adsorption of one substance can disrupt the metabolism of others, thereby creating the necessary conditions for hidden pathologies that cannot be detected in blood plasma analysis. The practical importance of the adsorption-transport function of erythrocytes has been considered in relation to diabetes and atherosclerosis [1].Adsorption of proteins in erythrocytes affects the deformation and rheology of blood, and the adsorbed part of the protein is a reserve for emergency replenishment of proteins in the plasma [2]. It was found that the process of transporting organic substances in erythrocytes is more variable and demonstrative compared to the corresponding plasma indicators. It has been proven to regulate transport of substances in erythrocytes and maintain adsorption due to the activity of physicochemical bonds of hemoglobin inside erythrocytes [3]. Thus, there is a second important function of erythrocytes - adsorption-transport function, which plays an important role in the processes of rapid and selective entry of substances into the exchange layer of blood capillaries [4]. The passage of erythrocytes through the narrow parts of the capillaries ensures mechanical replacement of molecules adsorbed on erythrocytes with substances in the wall exchange layer. Differences in the ability of substances to be adsorbed on the surface of erythrocytes made it possible to divide them into weakly, moderately and strongly adsorbed types.
2. Purpose of the Research
- The goal of our scientific work is to study the sorption capacity of erythrocytes in erythrocyte masses prepared in different forms.
3. Material and Methods
- Due to the fact that the research was conducted only on the blood of donors, blood was taken from a total of 48 male donors of Uzbek nationality aged 21-62 at the Republican Blood Transfusion Center (Tashkent, Republic of Uzbekistan). Blood donors are from almost all regions of the republic, and among them there are both urban and rural residents. Donors were informed that their biological material would be used in the intended research study and their consent was obtained.In the general analysis of the blood of all studied donors, the following were determined: the amount of erythrocytes (RBC), leukocytes (WBC), platelets (PLT) and hemoglobin (Hb). The erythrocyte mass was separated from the collected blood, washed erythrocytes were obtained from one part, one part was frozen, one part was irradiated, and another part was leukofiltered. These procedures were carried out together with specialists of the Republican Blood Transfusion Center. The ability of erythrocytes to absorb endogenous substances such as total protein, albumin, glucose, cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein, urea and creatinine was studied. The content of blood plasma was determined using an automatic biochemical analyzer HumaStar 100 (Federal Republic of Germany).To determine the rate of absorption of erythrocytes, the blood collected in a test tube with heparin was centrifuged at 3000 rpm for 10 minutes, and the plasma was separated.Work progress:1. Total protein (g/l), albumin (g/l), glucose (mmol/l), cholesterol (mmol/l), triglycerides (mmol/l) in the isolated plasma using the automatic biochemical analyzer HumaStar 100 (Federal Republic of Germany), urea (mmol/l) and creatinine (μmol/l) were determined.2. The erythrocyte masses were mixed with plasma in a ratio of 1:1, mixed carefully and left at room temperature for 10 minutes.3. Then the tubes were again centrifuged at 3000 rpm. Plasma was isolated and the above endogenous substances were determined again in a biochemical analyzer.4. To determine the rate of absorption of erythrocytes, we divided the difference of results 1 and 2 by 10 and found the change in concentration in 1 minute.
4. Results and Discussion
- Our research to study the sorption capacity of erythrocytes in erythrocyte masses prepared in different forms showed that the sorption capacity of washed erythrocytes was 3 times higher than the control (Table 1). That is, control erythrocytes adsorbed 489 mg of protein per 1 minute, while washed erythrocytes adsorbed 1440 mg of protein per 1 minute. The albumin sorption capacity of the washed erythrocytes was also 1.3 times higher than the control (900 mg/min versus 711 mg/min in the control). Glucose uptake in controls was 148 μmol/min, whereas washed erythrocytes absorbed 838 μmol/min of glucose.
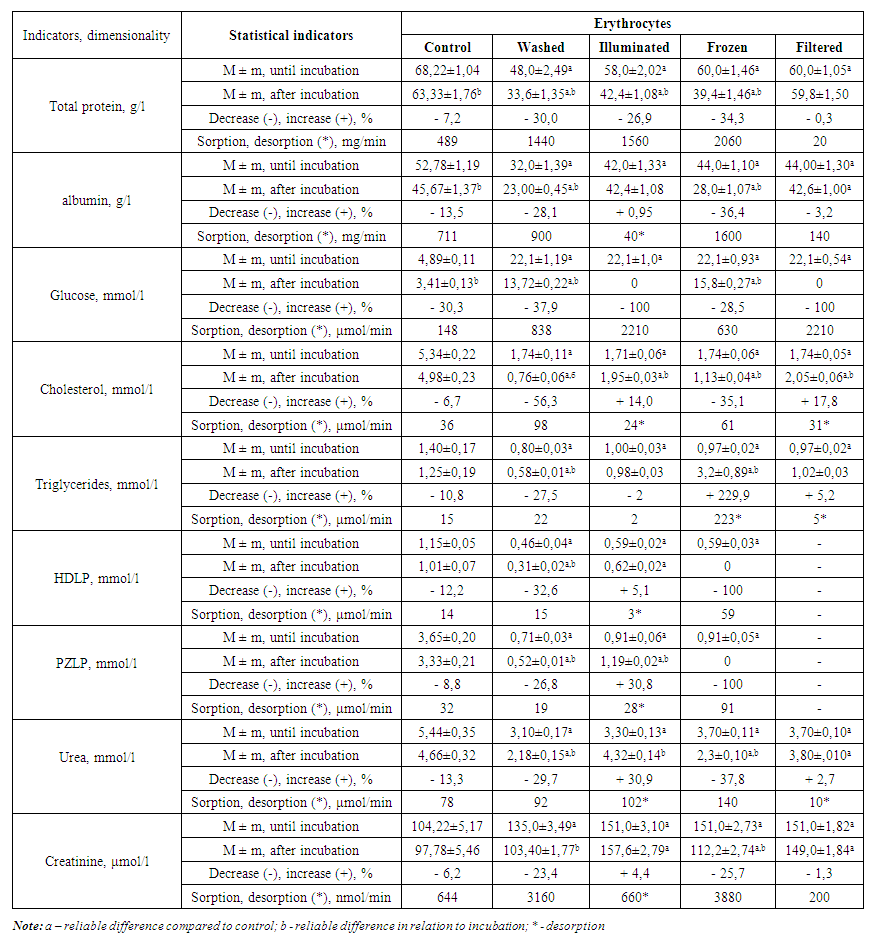 | Table 1. The ability of erythrocytes in the erythrocyte mass to absorb various substances from the blood plasma |
5. Conclusions
- The obtained results indicate that certain changes occur in the cells during the preparation of erythrocyte-preserving drugs. These results lead to the conclusion that in the future, the use of erythrocyte mass erythrocytes prepared by different methods should be approached taking into account their ability to absorb substances.Based on the analysis of the obtained results, we present the following conclusions:1. In the washed erythrocytes, significant changes in the ability to absorb various endogenous substances are observed. In this case, the rate of sorption of endogenous substances mainly increases.2. In irradiated erythrocytes, there are significant changes in the ability to absorb various endogenous substances. In this case, not only sorption of endogenous substances, but also reverse processes, i.e. desorption, are observed.3. Big changes are also observed in frozen erythrocytes in their ability to absorb various endogenous substances. In this case, the ability to sorb endogenous substances is observed for all substances, and for triglycerides, the reverse process, i.e., desorption, is also observed.4. In leukofiltered erythrocytes, great changes are observed in the ability to absorb various endogenous substances. In this case, erythrocytes almost lose their ability to absorb many endogenous substances.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML