-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Life Sciences
p-ISSN: 2163-1387 e-ISSN: 2163-1395
2012; 2(3): 68-74
doi: 10.5932/j.als.20120203.05
Effect of Season on Catch rate, Diet and Aspects of Reproduction of Clarias gariepinus (Teleostei: Clariidae) in a Tropical Waterfalls
Gabriel U. Ikpi1, Adetola Jenyo-Oni2, Benedict O. Offem1
1Department of Wildlife and Fisheries Management, Faculty of Agriculture and Forestry, University of Ibadan, Nigeria
22Department of Wildlife and Fisheries Management, Faculty of Agriculture and Forestry, University of Ibadan, Nigeria
Correspondence to: Benedict O. Offem, Department of Wildlife and Fisheries Management, Faculty of Agriculture and Forestry, University of Ibadan, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The African catfish Clarias gariepinus (Burchell 1822) are highly valued food-fish and are among the dominant fishes of commercial catches in major rivers in Africa. The aim of the present study was to provide data on reproductive biology, diet habits, length–weight relationship and condition factor that can be useful for conservation and propagation of this species. The study was carried out over 24 months in the three reaches of the 200 km- long of Agbokum waterfalls, Nigeria. The influence of season on catch rate, reproduction and diet was determined from catch samples. There was a significant difference in the catch rate between the dry and wet seasons. Peak catch occurred during the early and late rains. The peak breeding period was June - July. The fish were omnivorous, with trophic flexibility being related to food availability. The condition of the fish was better downstream of the waterfalls than the other two reaches, but declined during dry season and improved during early and late rains. Therefore downstream reaches during wet season are significant in the fisheries studies of waterfalls
Keywords: Waterfalls, Catch Rate, Fecundity, Diet Habit, Condition Factor, Season
Article Outline
1. Introduction
- Clarias gariepinus belong to the family Clariidae and occur in most freshwater bodies of South East Asia and Africa where they constitute a significant component of the catches. The highest genetic diversity is found on the African continent where some 14 genera have been reported[1] against two in Southeast Asia. They have high market value in Nigeria and the world at large, and a vital source of vitamins and protein which are highly digestible[2]. They are very strong and easily adapted to their environment; they are inexpensive in term of the cost of production when cultured. They live in freshwater lakes, rivers, swamps, as well as human-made water bodies, such as ponds or even urban sewer systems[3]. Clarias gariepinus commonly refer to as African Catfish is a large, eel-like usually of dark gray or black coloration on the back, fading to a white belly. It has a slender body, a flat bony head and abroad terminal mouth with four pairs of barbell[4]. In both continents Clarias gariepinus are of great economic importance as food fish and vital in the sustainability of aquaculture due to their attributes[5] which include; abil ity to withstand handling stress, disease resistance, high growth rate, yield potential, fecundity and palatability[6]. There is acute reduction of these species in inland waters in Nigeria because of the over-exploitative nature of indigenous fishers that destroys the habitat and fisheries resources[7]. Knowledge on their biology is important for rational utilization of stock. An effort by the Nigerian government to conserve and propagate these species through fisheries regulation and fish breeding is being hindered because of the little information available on the ecology of these species in Nigerian waters. Most of the research works are limited to the reproductive biology of C. gariepinus[8, 3, 10-13]. No work had been undertaken on the ecological studies of C. gariepinus in Waterfalls. This paper therefore provides information on the ecological influence of season on the distribution catch rate, diet and reproduction of C. gariepinus in waterfalls of Southeastern Nigeria. These data will form the bases for management strategy of this species in waterfalls in Africa.
2. Materials and Methods
- Study area. The study area is Agbokim Waterfalls in Cross River State, Nigeria (Figure 1).
 | Figure 1. Map of Cross River State showing Agbokim Waterfalls |
3. Results
- Monthly variation in catches revealed that peak catch was biphasic occurring in the early (May-July) and late wet months (November-January) in all the reaches (Figure 2A-B). Catches of the juvenile fish was significantly higher (p<0.001) in the early wet months (May-July). The maturity curve showed that 50% of the males matured at 16.5cm TL and females at length 14.6cm (Figure 3). GSI varied from 8.9 to 20.6 in female fish and from 4.2 to 12.5 in male fish and was higher in the wet season than dry (p<0.05). Monthly changes in the GSI of C. gariepinus females and males in consecutive months revealed that both values were very low during September to February and highest in May (Figure 4).
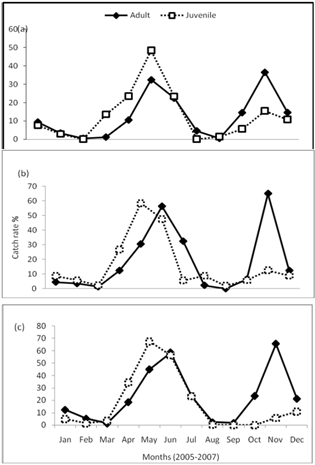 | Figure 2. Percentage monthly catch rates of adult and juvenile of gariepinus in the Agbokum waterfalls: (a) upstream, (b) midstream and (c) downstream reaches |
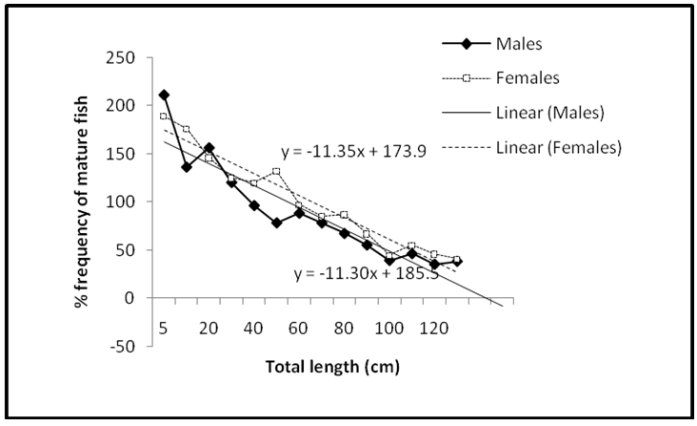 | Figure 3. percentage occurrence of mature males and females C. gariepinus at different lengths |
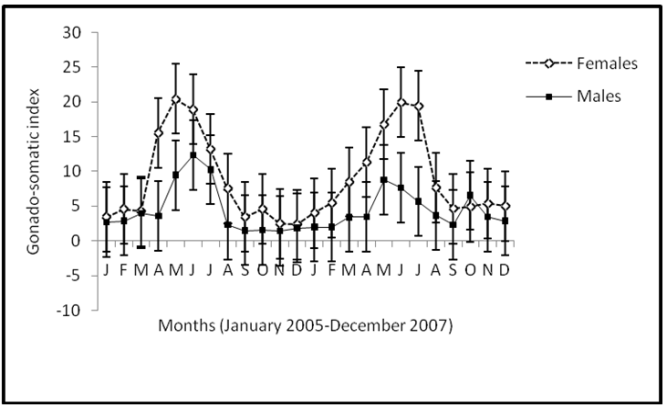 | Figure 4. Dynamics in the gonadosomatic index (GSI) of the male and female C. gariepinus in Agbokum waterfalls |
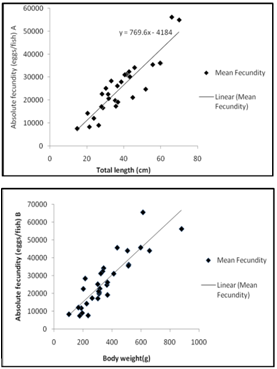 | Figure 5. (a) Fecundity-length relationship and (b) fecundity-weight relationship |
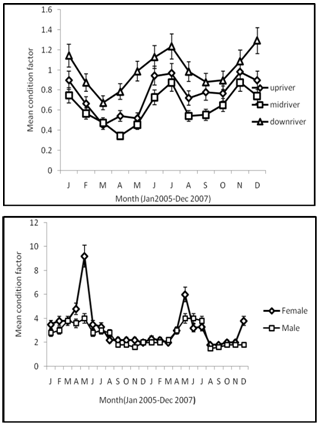 | Figure 6. Monthly mean variation of condition factor of male and female C. gariepinus combined from Agbokim waterfalls by (a) reach and (b) sex |
|
- The most frequent food item found in the African catfish in all the reaches were fish prey and fish remains constituting 65 percent of the diet in the wet season and <10 percent in the dry season (Table 1). Food objects, habitually, dominating the dry season, included crustaceans, insects, and plant materials, constituting 79% of the diet. Indices of the diet composition showed that Gut Repletion Index (GRI) was 85% in both wet and dry season and 67% and 92% in the day and night, respectively. The least prey diversity was found in dry season diet (H =4.7 and 0.5 for wet and dry seasons respectively). Diel pattern in the consumption of food items by C. gariepinus showed eleven food types ingested during the night while seven types were taken during the day (Table 2). The occurrence of all items except plant materials was significantly higher (P < 0.05) in the night catches than the day.
4. Discussion
- The seasonal variation in the catch rate of Clarias gariepinus as indicated in the analysis of variance support the notion that various fish communities show non-random patterns in composition over time[29, 30]. Seasonal variation in catch rate may be as result of the period of courtship, during which and resting take place and the species withdraws to suitable and safe areas for spawning[31]. Peak of GSI for this species in April-May was confirmed by the largest frequency of the matured phases during this period; characterizing a single annual spawning, and synchronic ovarian development. By June/July, spawning was over, as indicated by the decrease in GSI and the presence of freshly spent ovaries. It has been reported[32] that the final trigger of spawning in Clarias species is caused by a rise in water level due to rainfall; which commenced in the study area between April and June[33, 34] all found out that in some localities catfishes present a single annual spawning period corresponding to the late dry season or early wet season, associated with high water temperature. Our findings indicate that, for captive breeding programs gravid fishes are available in the wild during April-August. During October to January most ovaries were spent, indicating that the spawning period was over. Therefore, from the point of view of fisheries management the best fishing period for the species in the Agbokom waterfalls is October- January because gravid fish are almost absent during the period. Although the juveniles are abundant during the period, with the use of appropriate fishing methods and management practices such as regulated mesh size (>62.8mm stretched mesh), juvenile overfishing could be avoided, and only the table sizes will be harvested. A closed season, or less intense fishing, during April to September, when gravid C. gariepinus are abundant, would help to conserve the natural stocks, by allowing the fish to breed at least once in their lifetime. The relative fecundity range of 453-678 eggs cm-1 recorded in this study was comparatively high compared to observations from other clariid catfishes. 308-403 eggs cm-1 from C. gariepinus had been recorded in Hardap dam, Namibia[8] and 203-278 eggs cm-1 in Cross River[35] while 443-498 eggs cm-1 were reported in Opa reservoir, Nigeria[9]. This shows that C. gariepinus in waterfalls may be more fecund than those in other fresh water bodies. Peak season in fecundity of these species which coincides with onset of rains and the rising flood have also been observed in the River Niger[36, 37, 38]. All concluded that most tropical fishes breed on the rising flood thus allowing the juveniles to take full advantage of the flooded banks for feeding, while being protected from predation. There are indications that the intensity of flooding influences the reproductive success as stronger year classes have been noted from those years when particularly intense floods occur in the Kafue River and the Central Delta of the Niger, Senegal and Lake Chad (Stauch, Kainji Research Institute, Nig.( pers. comm.) Our data show that condition factor is highly correlated with GSI. However, with higher GSI the weight for length is bound to increase, irrespective of whether or not the food organisms are in good or bad condition. The higher condition factor obtained between June and July, and low values between August and October, marched findings reported[34] and the results attributed to spawning activities, which may have resulted from the accumulation of fats and ripe gonads carried by the mature adult females. The observation that 62% of the samples examined had condition factor above mean showed that the majority of fish in the population are in excellent condition[39]. The diet composition showed C. gariepinus to be an omnivorous predatory fish. The diet spectrum in which fish prey and crustaceans were the most abundant, could be closely marched with that obtained in Clarias gariepinus from Ubangui River, Central African Republic[40], in Lake Sibaya, South Africa[41], in Lake Kinneret, Israel[42] and in Southern Florida[43]. There is considerable seasonal variation in the feeding activity correlated with flood regime. During floods the release of nutrients, rapid growth of vegetation and the increased availability of other sources such as seeds, young shoots, leaves and molluscs form the basis for a particularly intense feeding activity[44]. The diet generally reflects the seasonal distribution of macro-invertebrates and fish prey in the environment[45, 46]. Presence of plant materials in the diet of this species had been reported[47, 48] and it was suggested that the frequent occurrence, especially during dry season, could be a survival strategy in times of scarcity of prey. The gut repletion index of 85 percent indicates that the fish is a regular feeder. High values of other indices such as Food Richness and Diet breadth showed that the species exhibits trophic flexibility, an ecological advantage that enabled the fish to switch from one food category to another in response to fluctuation in their abundance[49]. The diel pattern of feeding, where C. gariepinus completely ignored fish prey during the day and fed mainly on invertebrates and plants, was also observed[41]. He attributed this behavior to its inability to catch prey fish in daylight due to its slow, methodical, predatory tactics. The generally higher diet indices recorded from night catches in this study had also been reported[43] and suggested that the species is more active at night.
5. Conclusions
- The species exhibits trophic flexibility, an ecological advantage that enabled the fish to switch from one food category to another in response to fluctuation in their abundance. Majority of fish in the population are therefore in excellent condition. A closed season, or less intense fishing, during April to September, when gravid C. gariepinus are abundant, would help to conserve the natural stocks, by allowing the fish to breed at least once in their lifetime.
ACKNOWLEDGEMENTS
- The authors are grateful to Senate of Cross River University of Technology, Nigeria for the Grant to support in this project.
References
| [1] | Teugels, G. G., 1986a, A systematic revision of the African species of the genus Clarias (Pisces: Clariidae) Museum Africa Centre, Belgium. pp3-72 |
| [2] | Haruna B. G., 2003, Sex ratio and fecundity of Clarias gariepinus in Opa Reservoir, Ile-Ife, Nigeria. Proceeding of annual conference of Fish Soc Nigeria Proc. FISON, 12(7), 122-130. |
| [3] | Teugels, G.G. 1982, Prelimnary data of a systamatic outline of the African species of Genus Clarias (Pisces: Clariidae), J. Nat. Hist. 95, 11-28. |
| [4] | van den Bossche, J.P. and Bernacsek, G. M., 1990, Source book for the inland fishery resources of Africa. 2. CIFA Technical Paper 18.2, FAO, Rome Italy, p411 |
| [5] | Aluko, P. O. and Shaba, M. 1999, Intra- and inter-specific hybridization studies between exotic Clarias gariepinus and two indigenous Clariid species. Nigerian J. Gen. 14, 59-63. |
| [6] | Viser, S. A. 1970, Kainji, a Nigerian man-made lake. Kainji Lake studies. Nigerian Inst Soc Econ Res, Ibadan. p126. |
| [7] | Gaigher, I. G., 1977, Reproduction in the catfish (Clarias gariepinus) in the Hardap dam,South West Africa. Madoqua 10: 52-59 |
| [8] | Abayomi, R. E. and Arowomo A. F., 1996, Sex ratio and fecundity of Clarias gariepinus in Opa Reservoir, Ile-Ife, Nigeria. Proceeding of annual conference of Fish Soc Nigeria Proc. FISON, 12(7), 122-130. |
| [9] | Ugumba, O. A. and Ugumba, A. A., 1988, Induced ovulation, artificial spawning, survival and early growth of larvae of the African catfish, C. gariepinus. In:Otubusin SO, Ezeri GNO, |
| [10] | Machiels, M. A. M, Herken, A. M., 1985, Growth rate, feed utilization and energy metabolism of the African catfish Clarias gariepinus as affected by protein and energy content. Aquacult 46,19-23 |
| [11] | Ayinla P.O. and Nwadukwe, D.Y. 1999, On length-weight relationship. Fishbyte section 5, 11-13. |
| [12] | Fagbenro, O. A., 1993, Studies on the biology and aquaculture potential of feral catfish Heterobranchus bidorsalis (Geoffrey St. Hilaire 1809) (Clariidae). Trop. Zool. 6, 67-79 |
| [13] | Teugels, G. G., Reid, G. Mc. G. and King, R. P., 1992, Fishes of the Cross River Basin (Cameroon - Nigeria). Taxonomy, Zoogeograhpy, Musee Royal Del Afrique-Centrale, Tevuren, Belgique. Ann Sci Zoolog 260:132. |
| [14] | Fischer, W., and Bianchi, G., 1984. Species identification sheets for fishery area. FAO, Rome, pp34-47 |
| [15] | Idodo-Umeh, G., 2003, Fresh water fishes of Nigeria. Taxonomy, ecological notes, diets and utilization. Idodo-Umeh Publishers Limited. p232 |
| [16] | Olaosebikan, B. D. and Raji, A., 1988, Field guide to Nigerian freshwater fishes. Federal College of Freshwater Fishes (FCFF), New Bussa, Niger State, Nigeria. 106pp. |
| [17] | Holden, M. and Reed, W., 1972, West African fresh water fish. Longman Group Ltd, London, 68pp. |
| [18] | Buije, A.D. and Houthuijzen, R. P., 1992, Piscivory, growth, and size- selective mortality of age 0 pikeperch (Stizostedion lucioperca). Can. J. Fish. Aquat. Sci., 49, 894 – 902. |
| [19] | Windell JT and Bowen M (1978) Food analysis and weight of digestion met hod of studying food consumed. In: Bagenal T (ed) Fish production in fresh water. Oxford. pp197-201. |
| [20] | Hyslop, E. J. 1980, Stomach content Analysis: A review of methods and their application. J. Fish. Biol. 17,111-129. |
| [21] | Simpson, A.C., 1951, The fecundity of plaice. U.K. Ministry of Agriculture and Fish Food Invest Serv 217: 3-27. |
| [22] | June, F. C. 1953, Spawning of yellwfin tuna in Hawaiian waters. Fishery Bulletin 54,47 –61. |
| [23] | Ricker, W.E. 1975. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Bd. Can. 191: 209-210. |
| [24] | Andrews D. G. and Matsuda O.U., 1975, Length-weight parameters and condition factor of two West African prawsn. Rev. Hydrobiol. Trop. 27 (2), 121-129. |
| [25] | Hynes, H. B. N., 1970, The ecology of running waters. Liverpool, Liverpool University press. 555 pp. |
| [26] | Steel, R.G.D, Torrie, J. H. 1980, Principles and procedures of statistics. NewYork McGraw Hill Book company. New York p 063. |
| [27] | Lewontin,R.C.1966, On the measurement of relative variability. System Zool 15, 141- 142. |
| [28] | Jackson, D. A, Harvey, H. H, 1997, Quantitative and qualitative sampling of lake fish communities. Can. J. Fish Aquat. Sci. 54, 2807-2813. |
| [29] | Connor, E. F., Simberloff, D. 1979,The assemblage of species communities. Ecol60,11- 32. |
| [30] | Bruton, M. N. 1979a, The breeding biology and early development of Clarias gariepinus in Lake Sibaya, South Africa. Trans. Zool Soc London 35, 1-45. |
| [31] | De Graaf J. G., Galemoni F. and Banzoussi B. 1995, Recruitment control of Nile tilapia, Oreochromis niloticus, by the African catfish, Clarias gariepinus. Aquaculture Research 30, 25-36. |
| [32] | Dmitincko, E. M. 1970, Reproduction of the sea catfish (Arius thalassinus, Ruppel) in the Arabian sea. J Appl Ichthyol 10, 361-364. |
| [33] | Rimmer, M. A. and Merrick, J. R. 1983, A review of the reproduction and development in the fork-tailed catfishes (Ariidae). J Limn Soc London 107, 41-50. |
| [34] | Offem, B. O., Akebgejo-Samsons, Y. and Omoniyi, I. T., 2010, Aspects of ecology of Clarias anguillaris (Teleostei: Clariiae) in the Cross River, Nigeria. Turk J Fish and Aquat Sci. 10, 101-109. |
| [35] | van der Waal B. C. W., 1975, Observations on the breeding habits of African catfish, Clarias gariepinus (Burchell). J. Fish Biol. 48, 6-10. |
| [36] | Harding, D., 1966, Lake Kariba: the hydrology and development of fisheries. In: Lowe McConnell RH (ed) Manmade lakes. Academic Press, London, pp65-66 |
| [37] | Blache, J. 1964, Les poissons du Basin de Tchad et du Basin adjacent du mayo kabbi. Etude systematique et biologioue, Tome. ORSTROM 4,11483 |
| [38] | Fagade, S. O., Adebisi, A. A. and Atanda, A. N., 1984, The breeding cycle of Clarias lazera.Hydrobiol. 100, 493-500. |
| [39] | Micha, J. C. 1973, Etude des populations piscecoles de I’Ubangui et tentative de selectionet d’adaptation dequelques especes a l’etang de pisciculture. Centre Technique Forestiere Tropical, Nogent Sur Marne, p100 |
| [40] | Spatura, P., Viveen, W. J. A. R. and Gophen, M. 1987, Food composition of Clarias gariepinus in lake Kinneret, Israel. Hydrobiol. 144, 17-23. |
| [41] | Robbins, H. R. 2004, Description: walking catfish - Clarias batrachus. US Geological Survey, Florida, p7. |
| [42] | Blache, J. 1964. les pessions du basin du Tchad et du basin adjacent du mayo kebbi. Men ORSTOM 4.2 : 48 3pp. |
| [43] | Wooton, R. J., 1979, The effect of size of food ration on egg production in the femalethree-spined stickleback,Gasterosteus aculeatus. J Fish Biol 5:89-96. |
| [44] | Madu, C.T., Fodike, E. B. C and Ita. F. 1991, Food and feeding habits of hatchlings and the mudfish Clarias gariepinus. J Aquat Sci 5: 27-32. |
| [45] | Courtenay, W.R., 1970, Florida’s walking catfish. Words Nat Sci Bull 10, 1-6. |
| [46] | Courtenay, W. R., Sahlman, W. W., Miley, I. I. and Herema, D.J., 1974, Exotic fishes in fresh and brackish waters of Florida. Biol. Conserv. 6, 294-340. |
| [47] | Clay, D. 1979, Population biology, groth and feeding of African catfish, Clarias gariepinus, with special reference to juveniles and their importance to fish culture. Hydrobiol 87, 453-487. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML