-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Textile Science
p-ISSN: 2325-0119 e-ISSN: 2325-0100
2014; 3(1A): 1-5
doi:10.5923/s.textile.201401.01
Mechanically Robust and Antimicrobial Cotton Fibers Loaded with Silver Nanoparticles: Synthesized via Chinese Holly Plant Leaves
Naseeb Ullah1, Sohail Yasin2, Zamir Abro2, Lin Liu2, Qufu Wei1
1Key Laboratory of Eco-Textiles, Ministry of Education China, Jiangnan University, Wuxi, Jiangsu, 214122, China
2Key Laboratory of Advanced Materials and Textiles, Ministry of China, Zhejiang Sci-Tech University, Hangzhou, Zhejiang 310018, China
Both Naseeb Ullah and Sohail Yasin contribute equally to this work.
Correspondence to: Qufu Wei, Key Laboratory of Eco-Textiles, Ministry of Education China, Jiangnan University, Wuxi, Jiangsu, 214122, China.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
In this work we investigated cost effective and environment friendly antibacterial properties of cotton fibers loaded with silver nanoparticles (AgNPs) synthesized from natural Chinese Holly plant extracts as a reducing and capping agent. The formation of AgNPs from Chinese Holly plant leaves extract was observed by UV–vis spectrophotometer and was found to be <100nm in size confirmed by electron microscopy. The antimicrobial properties of cotton fibers loaded with silver nanoparticles was evaluated against gram-negative Escherichia coli (E.coli) bacteria. The results showed great antibacterial properties by merging 1.5-4.5% of Chinese Holly leave extracts. These cotton fibers also showed fine antibacterial efficiency after several washings making it suitable for medical applications with an ease.
Keywords: Nanoparticles, Cotton fibers, Antibacterial properties, Chinese holly leaves
Cite this paper: Naseeb Ullah, Sohail Yasin, Zamir Abro, Lin Liu, Qufu Wei, Mechanically Robust and Antimicrobial Cotton Fibers Loaded with Silver Nanoparticles: Synthesized via Chinese Holly Plant Leaves, International Journal of Textile Science, Vol. 3 No. 1A, 2014, pp. 1-5. doi: 10.5923/s.textile.201401.01.
Article Outline
1. Introduction
- Due to the recent development in novel technologies regarding synthesis of metal nanoparticles has gained popularity, research related to nanoparticles is expanding because of their vast applications. Usually, nanoparticles are prepared by different chemical methods which are mostly not eco-friendly. This study reports a fast and suitable method of producing silver nanoparticles from Chinese Holly plant leaves and silver nitrate respectively, focusing economical approach for synthesis of AgNPs. As cotton is cellulosic in nature, it makes it more prone towards bacterial growth. So, the cotton fibers are treated with different chemicals to get antibacterial cotton textiles [1-2]. Synthetic methods for the preparation of silver nanoparticles, mostly chemical reducing agents are linked with environmental toxicity or health hazards. Therefore, new development in synthesis of silver nanoparticles from natural extracts is considered to be the most appropriate method keeping the environmental issues accounted [3-5]. Though green synthesis of nanoparticles using plant extracts such as Alfalfa, Aloe Vera, Cinnamomum camphora, Neem, Emblica officianalis, Lemongrass, and Marri has been accounted in recent researches, but the complete potential of the plants used as reducing agents for the synthesis of nanoparticles is yet to be discovered [6-12].This study engages with the integration of silver nanoparticles on cotton fibers synthesized via Chinese Holly leaves extract. The cotton fibers having silver nanoparticles also attained great mechanical properties as well, which is due to the functional groups present in extracts of Chinese Holly leaves. Chinese Holly plant (Ilex Cornuta) is a species of the genus Ilex in Aquifoliaceae plant family. It is native to and abundantly found in Southern China. The Chinese Holly (Ilex Cornuta) is valued in horticulture for its attractive and distinctive glance with red berries. The Chinese Holly plant (Ilex Cornuta) is famously used for tonic, contraceptive, febrifuge and for carminative purposes. Particularly, some say it gives strength to backbones and knees; the whole plant made into soup, which is used in fever, joints and lower back pain [13-16].
2. Experimental
2.1. Materials
- The Ilex Cornuta leaves were collected from their trees available in the Xiasha campus of Zhejiang Sci-Tech University, Hangzhou, China. Silver nitrate (AgNO3) was purchase from Strem Chemicals, Inc. (USA). E. coli (ATCC 25922) and S. aureus (ATCC 6538) strains were taken from the College of Life Sciences, Zhejiang Sci-Tech University. Commercial washing powder “Tide” was used for washing durability tests. Cotton fibers with 1cm length were used for antibacterial activities. Distilled water was used throughout the experiments.
2.2. Preparation of the Ilex Cornuta Leaves Extract
- The extract solution was prepared by boiling 1.5, 3 and 4.5 grams of leaves in an Erlenmeyer flask with 100ml of distilled water for 10 minutes at 100ºC followed by filtration and stored at 4ºC.
2.3. Synthesis of Silver Nanoparticles
- In usual experiment 5ml of the fresh leaves extract was added to a conical flask containing 5ml of 1mM aqueous AgNO3 solution at room temperature. The silver ions were reduced to silver nanoparticles within 2-3mins by the Chinese Holly plant leaves extract. The quick conversion of solution color showed the formation of silver nanoparticles by observing the color change from colorless to yellowish-brown color.
2.4. Antibacterial Activity
- The antibacterial activity against E. coli and S. aureus was evaluated as an index after 8h of bacterial culture, which was calculated as the percent of bacterial reduction using the following equation;
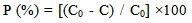 | (1) |
2.5. Washing Durability Test
- The laundering durability of antibacterial activities was evaluated using the AgNPs deposited cotton fibers prepared for 30 minutes were washed in the bath containing 0.5% “Tide” washing powder at liquor-to-fiber ratio of 300:1. After 30 minutes of washing at 40oC, the fibers were rinsed at temperature and dried at 37oC. The antibacterial activity of cotton fibers after 0, 2, 3, and 5 washing cycles was determined according to the standard method (dynamic shake flask method) (ASTM-E 2149: 2001, Standard test method for determining the antibacterial activity of immobilized antibacterial agents under dynamic contact conditions).
2.6. Mechanical Properties
- The mechanical properties cotton fibers with and without silver nanoparticles were verified by using INSTRON 3369 Universal Testing Machine using 10kg load cell. The cotton fibers with cm length were tested three times and the average values were taken.
3. Results and Discussion
- Generally, the methods followed to reduce silver ions to silver nanoparticles, such as; Ultra Violet or Gamma radiation, photochemical, ultrasound or other chemicals are either very expensive or chemically toxic to environment somehow. Therefore, the main purpose of this research was to produce silver nanoparticles without any harmful chemicals relatively using just natural eco-friendly leaves.
3.1. Characterization of AgNPs
- After the reaction of AgNO3 with Chinese Holly leaves extract, it was observed that the color of the solution was changed to yellowish brown due to excitation of surface Plasmon vibrations in the metal nanoparticles. It was observed in UV–visible spectrum (Fig. 1) that surface Plasmon resonance occurred in between 400-450nm indicating the clear formation of AgNPs. The conversion of Ag+ ions into silver nanoparticles was due to the reduction action of functional groups such as flavonoids present in Chinese Holly leaves (Ilex Cornuta), different types of flavonoids like; quercetin, hyperin, 3'-methoxydaidzin, isorhamnetin, formononetin, kaempferol etc. [18]. These flavonoids are essential for the reduction process of silver nanoparticles, further, the high molecular chains present in Chinese Holly leaves provide stabilization for the produced silver nanoparticles [19]. On UV-visible spectrum scale, absorption peaks at 438.41, 440.53 and 443.11nm were observed in 1.5%, 3% and 4.5%, respectively which is due to the increase in the concentration of Chinese Holly leaves.
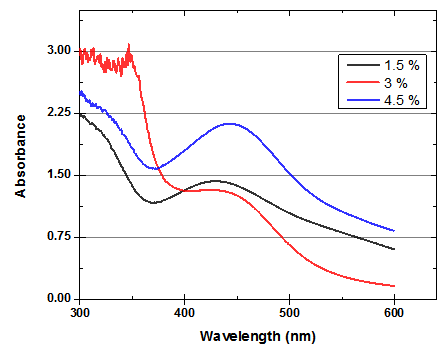 | Figure 1. UV-VIS absorption spectra of AgNPs synthesized from 1mM silver nitrate using 1.5, 3 and 4.5% of Chinese Holly leaves extract |
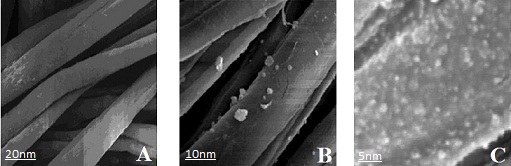
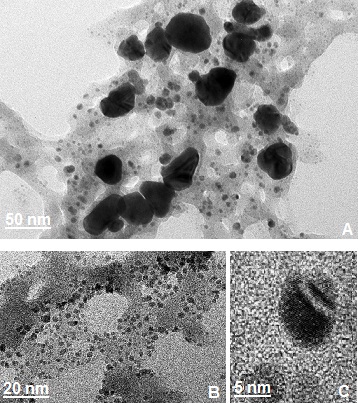 | Figure 2. TEM images of the silver nanoparticles synthesized by Chinese Holly plant leave extract showing different particle sizes. (a) 50nm scale, (b) 20nm scale, (c) 5nm scale |
3.2. Description of AgNPs Loaded on Fibers
- The loading of silver nanoparticles on cotton fibers were carried out by adding silver nitrate solution in leaves extracts broth, after instant reduction process the nanoparticles were stabilized by the polysaccharides. The cotton fibers were submerged into the stabilized silver nanoparticles solution for 24 hours. The silver nanoparticles settled on cotton fibers by polysaccharide or by some other functional groups present in the China Holly plant leaves extracts.To visually verify the formation of AgNPs on the cotton fibres, cotton fibres along with AgNPs loaded fibres were scanned under scanning electron microscopy (Fig. 3). The SEM images of control cotton fibres with AgNPs loaded fibers exhibits uniform neat plain spun structure and presence of AgNPs on the overall fibres at different scale levels.
3.3. Antibacterial Activity
- The antibacterial test was carried out by following the Standard SNV 195920-1992, stating a growth inhibition area of sample observed close to be >1 mm in size, the antibacterial properties are marked as ‘good’. And if the sample is totally rehabitated by the bacteria, the antibacterial property is marked as ‘not sufficient’. Different levels of antibacterial capacity are related to the dimension of the growth inhibition area around the samples [21]. The antibacterial activity of silver nanoparticles loaded cotton fibers was examined be measuring inhibition zone diameter by digital calliper (Table. 1). In present investigation the cotton fibers loaded with silver nanoparticles developed from Chinese Holly plant leaves have exhibited >1.5mm inhibition zone nearly in all the cases. All title and author details must be in single-column format and must be centered.
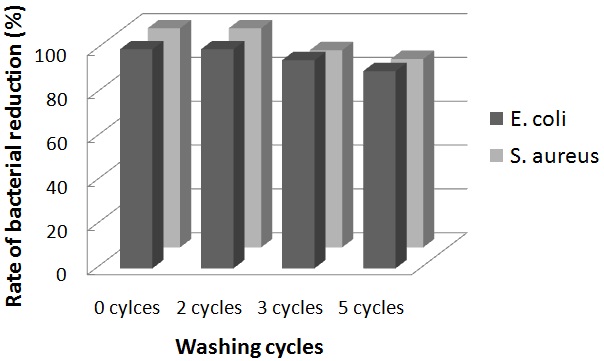 | Figure 4. Laundry durability of AgNPs loaded cotton fibers against E. coli and S. aureus |
| |||||||||||||||||||||||||||||||||||||
4. Conclusions
- In the field of nanotechnology development of an environment friendly and toxic free process for the synthesis of silver nanoparticles is vital. This study proudly defines a low cost and organic process for producing nanoparticles by using Chinese Holly plant leave extracts as a reducing agent. The results indicated that silver nanoparticles loaded on cotton fibers by using 4.5% Chinese Holly plant leaves extracts exhibited greater reduction of E. coli growth. This is because of the formation of smaller size nanoparticles, as well as a fast release of AgNPs into the medium. Based on this study it is possible to use these cotton fibers for medical applications such as surgical clothes, wound dressing, antibacterial finishing and other textiles.
References
| [1] | P. N. Danese, Antibiofilm approaches: prevention of catheter colonization. Chem. Biol., 9, 873 (2002). |
| [2] | K. Lewis and A. M. Klibanov., Surpassing nature: rational design of sterile-surface materials. Trends Biotechnol., 23, 343 (2005). |
| [3] | H. J. Lee, S. Y. Yeo and S. H. Jeong., Antibacterial effect of nano- sized silver colloidal solution on textile fabrics. J. Mater. Sci., 38, 2199 (2003). |
| [4] | W. K. Son, J. H. Youk and W. H. Park., Antimicrobial cellulose acetate nanofibers containing silver nanoparticles. Carbohydr. Polym., 65, 430 (2006). |
| [5] | W. K. Son, J. H. Youk, T. S. Lee and W.H. Park., Preparation of Antimicrobial Ultrafine Cellulose Acetate Fibers with Silver Nanoparticles. Macromol. Rapid Commun., 25, 1632 (2004). |
| [6] | J. L. Gardea, J. G. Parsons, E. Gomez, J. Peralta, H. E. Troiani, P. Santiago et al., Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett., 2, 397 (2002). |
| [7] | J. L. Gardea, E. Gomez, J. Peralta, J. G. Parsons, H. E. Troiani, M., Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Langmuir., 13, 1357 (2003). |
| [8] | S. P. Chandran, M. Chaudhary, R. Pasricha, A. Ahmad, and M. Sastry., synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog., 22, 577 (2006). |
| [9] | J. Huang, Q. Li, D. Sun, Y. Lu, Y. Su, X. Yang et al., Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanobiotechnol., 18, 105104 (2007). |
| [10] | B. Ankamwar, D. Chinmay, A. Absar and S. Murali, J. Nanosci., Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, Their Phase Transfer and Transmetallation in an organic solution. Nanotechnol., 10, 1665 (2005). |
| [11] | S. S. Shankar, A. Rai, B. Ankamwar, A. Singh, A. Ahmad and M. Sastry., Biological synthesis of triangular gold nanoprisms. NatMater., 3, 482 (2004). |
| [12] | B. Ankamwar, M. Chaudhary, and S. Murali., Gold nano-triangles biologically synthesized using Tamarind leaf extract and potential application in vapor sensing. Synth. React. Inorg. Metal-Org Nanometal. Chem., 35, 19 (2005). |
| [13] | Duke. J. A. and Ayensu. E. S. Medicinal Plants of China, Feddes Repertorium., 98, 398 (1987). |
| [14] | Stuart. Rev. G. A. Chinese Materia Medica. Taipei. Southern Materials Centre. |
| [15] | David and Charles, Hillier Nurseries. The Hillier Manual of Trees and Shrubs., pp. 281, (1998). |
| [16] | R. Phillips and M. Rix, Shrubs, Macmillan., pp. 277, Pan Books (1994). |
| [17] | N. Durán, P. D. Marcato, G. I. H. De Souza, O. L. Alves, and E. Esposito, antibacterial effect of silver nanoparticles produced by fungal; process on textile fabrics and their effluent treatment. J. Biomed. Nanotechnol., 3, 203 (2007). |
| [18] | S. X. Zhou, Z. R. Yao, L. Jun, P. F. Tu, Chinese J. Nat. Medic., 10, 84 (2012). |
| [19] | E. M. Egorova, A. A. Revina., Synthesis of metallic nanoparticles in reverse micelles in the presence of quercetin. Colloids Surf. A., 1, 87 (2000). |
| [20] | S. H. Lim, S. M. Hudson., application of a fiber-reactive chitosan derivative to cotton fabric as an antimicrobial textile finish. Carbohydr. Polym., 56, 227 (2004). |
| [21] | M. Pollini, M. Russo, A. Licciulli, A. Sannino, A. Maffezzoli., Characterization of antibacterial silver coated yarns. J. Mater. Sci. Mater. Med., 20, 2361 (2009). |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML