-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Sports Science
p-ISSN: 2169-8759 e-ISSN: 2169-8791
2016; 6(1A): 36-49
doi:10.5923/s.sports.201601.07

Vertical Climbing for Rodent Resistance Training: a Discussion about Training Parameters
Walter Krause Neto1, Wellington de Assis Silva1, Adriano Polican Ciena2, Carlos Alberto Anaruma2, Eliane Florencio Gama1
1Laboratory of Morphoquantitative Studies and Immunohistochemistry, Physical Education Department, São Judas Tadeu University, São Paulo-SP, Brazil
2Laboratory of Morphology and Physical Activity, Physical Education Department, “Julio de Mesquita Filho” São Paulo State University, Rio Claro-SP, Brazil
Correspondence to: Walter Krause Neto, Laboratory of Morphoquantitative Studies and Immunohistochemistry, Physical Education Department, São Judas Tadeu University, São Paulo-SP, Brazil.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Since the 50s, methods and protocols of resistance training for human beings have been discussed. Different equipment is proposed for rodent resistance training seeking similarity to human practice. Many studies are compared without taking into consideration the animal adaptation period, training induced stress status, type of equipment structure and training protocols used. This review aimed to discuss factual issues about Vertical climbing equipment, protocols, strength testing, cellular and molecular responses from resistance trained rodents. We verified the existence of a variety of vertical training equipment and protocols. Many outcomes were studied and shown to be effective through ladder climbing experimentation. Even so, cellular and molecular responses might be quite different regarding voluntary and non-voluntary studies. Finally, it has been shown that there is still the need for a more accurate control of the variables of training, such as intensity and volume, the number of load testing sessions and familiarization process, and others, approaching its findings to data recorded in humans.
Keywords: Ladder training, Muscular hypertrophy, Volume, Intensity
Cite this paper: Walter Krause Neto, Wellington de Assis Silva, Adriano Polican Ciena, Carlos Alberto Anaruma, Eliane Florencio Gama, Vertical Climbing for Rodent Resistance Training: a Discussion about Training Parameters, International Journal of Sports Science, Vol. 6 No. 1A, 2016, pp. 36-49. doi: 10.5923/s.sports.201601.07.
Article Outline
1. Introduction
- In human beings, morphological and physiological adaptations are needed to achieve better training goals [1-3]. Increasing muscle strength is the desired outcome in a variety of physical rehabilitation therapies [4, 5]. Skeletal muscle hypertrophy explains the gains in muscle strength, making for such Resistance training (RT) the primary model for studying this phenomenon. In the past three decades, many different methods and equipment have been developed for animal RT research [6, 7]. Review studies reported and questioned the feasibility of studying the effects of RT through experimental models because of the difficulty of mimicking the human strength exercise for animals [4, 8]. Searching similarity, different training equipment were developed such as squat, water jumping and resistive treadmill [4, 7]. Recently, Krause Neto et al. [7] demonstrated that many researchers had used ladder climbing (LC) equipment, and it might reflect a similar biological response found in human strength training and outcomes related to various diseases [8]. Clearly, animal training need to conduct similarly to human training procedures, reflecting through it, a clear and accurate training response [8]. Therefore, animal training apparatus and methodology should be equally related to human physical training. Exercise adaptation, load testing, familiarization process, training volume, and intensity must be continually controlled. Nevertheless, these variables information are lacking and scientists not always controlled these notes in an animal study. Therefore, many experimental results might be questioned with basis on this reflection. RT is used as a form of exercise for rehabilitation, and many researchers used the vertical training models as a form of non-drug treatment strategy [9-11]. Even so, much different vertical training equipment was found in the literature, and this might turn difficult comparison among it.Beyond all information available on human strength training protocols and its outcome, it was not sure if maximum strength testing, protocol and training parameters must induce hypertrophy in rodent skeletal muscle system. Perhaps, maximum load testing appears to be crucial to prescribing a more appropriate training session and loading percentages. However, it is not clear if an animal, like a rodent, could achieve it maximum load through a unique strength testing session. For last, many questions must still be answered, such as: What is the proximity between human and animal maximum repetition loads (MR)? Could an animal do maximum load on climbing movement? How could the scientists be sure that this animal achieved it maximum testing load? During training sessions, should the animal climb the ladder to failure or not? How is possible to determine fatigue in rodents? What is the most appropriate training protocol for inducing muscular hypertrophy on experimental research? Thus, this study aimed to review the characteristics related to resistance training on vertical climbing equipment and discuss factual issues that compose rodent training, protocols and testing parameters. For last, we included a discussion about cellular and molecular responses induced by LC training.
1.1. Critical Discussion of the Theme
- For this study, we did a systematic review search on PubMed, Science Direct and Google Scholar database on September 20th, 2014. We used the following key words: “Ladder climbing”, “Ladder training”, “vertical training”, “resistance training”, “resistance exercise”, “strength training”, “strength exercise”, rodents, rat, mice, mouse and animals. After the initial search, we included studies that had as a primary goal to study the effects of resistance training, using the vertical training model, on the morphology and physiology of rodents. Then, we separated the items into two categories: muscle hypertrophy and other outcomes (data are presented in Tables 1 and 2).In recent decades, rodent models for physical training generated questions such as specificity of the biological response to physical stress [6]. Animal resistance training is much questioned in the literature by the lack of proximity to strength training done by humans. Much of this criticism came from the use of positive and negative reward [12-17]. There are several types of equipment to simulate the effects of RT in rodents such as squatting, water jumping, muscle ablation, ladder climbing, resistive treadmill and others [8, 14, 18-22]. Cholewa et al. [6] described the resistance exercises for rodents as volunteers and non-volunteers. According to these authors, vertical training model should be classified as voluntary, once the animal had sufficient conditions to climb the equipment after the adaptation period. Variations at both, equipment structure and training protocol, might result in uncertain outcomes. Also, the type of muscles involved in climbing work and the analysis of results could lead us to different muscle responses. Therefore, we began our discussion by describing the structure of the equipment and the evolution of the training method about vertical climbing models. For last, a critical discussion about training parameters is presented.
2. Description of Equipment Structure
2.1. Wire Mesh Cylinder
- Yarasheski, Lemon and Gilloteaux [22] proposed one of the first models of climbing training. Here, rats climbed a 40 cm vertical cylinder equipment (90°) carrying up a continuous load to the top, to receive food as a reward (Figure 1A). The rodent should climb the apparatus 20 times/5 days/week for eight weeks. Clearly, equipment was effective in stimulating muscle hypertrophy in rats.Years later, Duncan et al. [23] using a similar device, failed to demonstrate gains in muscular hypertrophy as presented by earlier work. This fact is easily explained once the analyzed muscles and training protocol were quite different between them. Also, Bennell et al. [12] failed to demonstrate an increase in lean body mass of rats, after ten weeks of training, even showing a significant increase in training load. These studies clearly demonstrated the diversity of results found this climbing model.
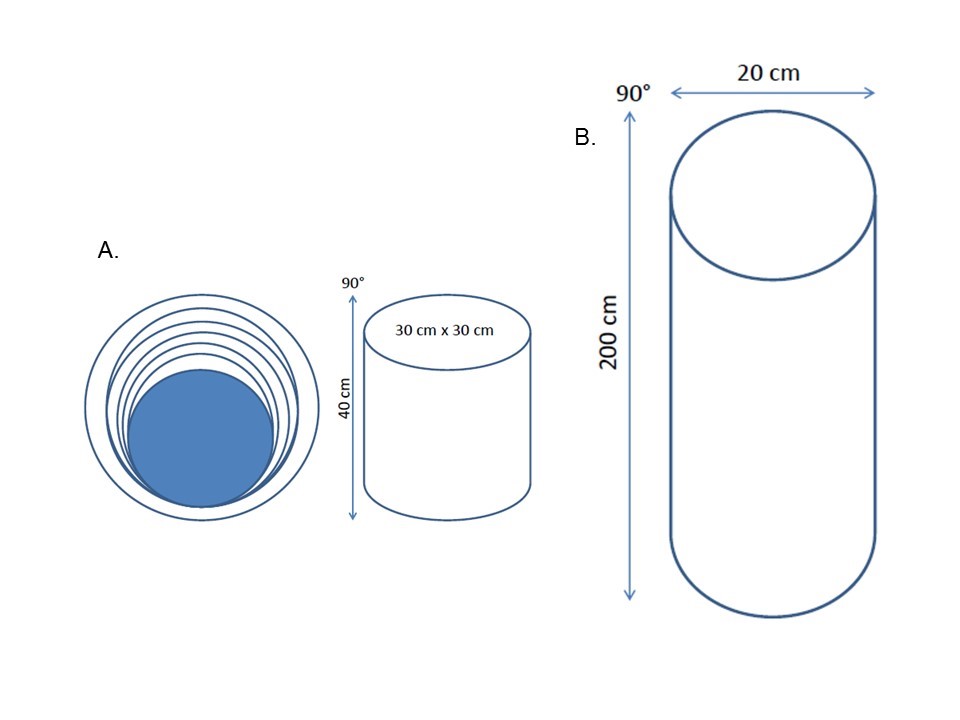 | Figure 1. Illustration of Wire mesh cylinder (A) and tower (B) equipment |
2.2. Wire Mesh Tower
- In subsequent years, similar equipment, however with a greater height, was proposed (Figure 1B). Notomi et al. [14] proposed a 200 cm cylindrical tower (90°) with metal steps attached to the rat´s cage. At this time, the animal should climb the tower to drink a bottle of water positioned at the top of the ladder. An important limitation of this model was shown by the absence of the bound load to the animal´s body. Thus, the principle of progressive overload was not applied. Also, lack of training control (number of climbs, average number of climbs per hour, the average time between each climb, and other) characterize a physical activity status and not physical training. Even so, authors demonstrated a bone mass increase in just four weeks of the experiment. Subsequently, other studies have shown that this RT model could aid in therapy for the prevention of anemia [24] and stimulate bone formation [25-27] in rats.
2.3. Ladder Climbing (LC)
- LC is the most RT used for rodent over the last years [7]. One of the first appearances of it came from the end of the 80s [20]. Currently, Hornberger and Farrar [28] described the most used and cited the type of RT in the literature (Figure 2). The equipment is 100 to 110 cm height, and it has 80 to 85 degrees of inclination. The artifact is easily constructed and provide low cost investment. According to authors, a rat performed 08 to 12 dynamic movements (repetitions) during each climb. Hornberger and Farrar [28] demonstrated the feasibility of Flexor Halux Longus (FHL) muscle hypertrophy and increase training overload over a few weeks.
2.4. Equipment´s Structure Adaptation
- Food or water deprivation may be factors that influence the adaptive response to RT. Perhaps, for this reason, models of wire mesh cylinder and tower are less used today (Table 1). Also, LC model presents the advantage of the animal does not need to receive reward or punishment during climbing. Finally, lots of muscle mass are mobilized to accomplish this task. This fact gives us the possibility of studying a broad range of systemic outcomes, such as diabetes mellitus, hypertension, and others. A disadvantage of all climbing equipment is the need to fasten the load at the animal's tail. This task induces a particular stress, and in many occasions, the load could detain the animal to climb the ladder by attaching it to the ladder steps. Thus, it is certain the need to start studying strategies to improve the physical structure of the equipment, and consequently to reduce the stress for rodents during the training period. Many types of equipment included in human training rooms are built through a system of pulleys. The possibility to adapt the LC equipment to a new pulley design becomes interesting, as it could reduce the stress on the animal. However, we must consider: what is the best strategy for rodent recover between each climb, once the ribbon tied to the animal's tail would continue to pull it out of the rest area? Possibly, a velcro tape tied to animal´s tail, with a musket lock, could be an attractive choice. Animal´s size should enable easy adaptation to the ladder and still allow us to tie the training load to the animal's tail. It is a consensus that these devices are built taking into consideration that animals may not be small. Wistar and Sprague-Dawley rats are widely used in vertical climbing studies, and those animals can increase training load and muscular resistance strength over a few weeks of training. Recently, the interest to adapt the ladder to smaller and more naturally active rodents such as mice is rising. Nevertheless, this animal´s size and aggressiveness might turn difficult to control training parameters.
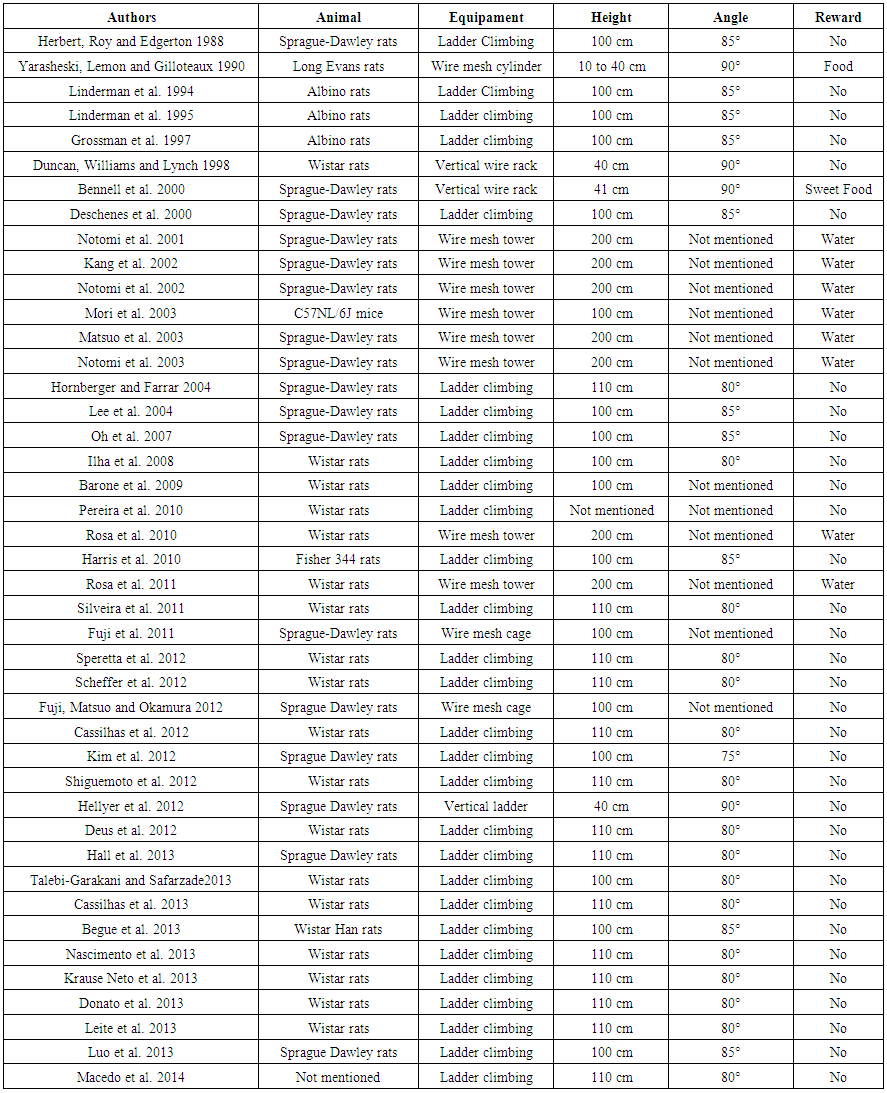 | Table 1. Vertical resistance training equipment characteristics |
2.5. Analyzis of Climbing Pattern
- It is widely known that muscle action, either concentric or eccentric, could interfere directly with the research results [29, 30]. Several human studies have shown that the combination of both movement phases must amplify the physiological and morphological responses to training [30]. However, when climbing pattern is analyzed, apparently all rodent performed concentric movements mostly. Once at the top, the researcher is the one responsible for putting it back on the base of the ladder to re-start the exercise. Thus, this single point of view might explain why some muscle types may and others not hypertrophy over this training model. Furthermore, the motor muscle characteristics must also be taken into consideration. Soleus and Plantaris muscles are widely researched. These muscles are highly recruited from the simple motion of LC [31, 32]. On the other hand, skeletal muscles such as Extensor Digitorum Longus (EDL) and Tibialis anterior (TA) are not prime movers during this type of exercise. Even so, some studies reported muscle hypertrophy of both Soleus and EDL [33]. This fact is intriguing, since Soleus muscle is a prime mover, and the EDL is not. The explanation might be simple once hindlimb plantar flexion is clearly displayed through climbing work (Soleus activation). Also, the movement of climbing might cause additional stress to secondary muscles such as EDL. Nevertheless, upper limb muscles are also recruited during climbing movement; however, very few papers were published regarding the analysis of such muscles. Nascimento et al. [34] showed muscular hypertrophy of the Triceps Brachialis (TB) of middle-aged rats after 16 weeks of training. Further studies should take into consideration the study of a wider broad of skeletal muscle types and not just only very few hindlimb muscles studied until now.
3. Training Protocols
- There are many training protocols for vertical climbing. As training for human, any change done in the variables involved in the prescription of the training protocol, even in rodent environment, can affect the animal´s adaptive response, and possibly, the results of the experiment [8].
3.1. Period of Adaptation and Equipment’s Familiarization
- The study of circadian rhythm is vast, and changes over this delicate biological control might cause significant behavioral and physiological changes [35]. Within our daily lives (human) the vast majority of the tasks are diurnal (light cycle). However, rodents are nocturnal creatures. Therefore, train a rodent during a light cycle might have different adaptive responses in comparison with their respective active period (dark). Just think about someone waking up at 2 or 3 o'clock in the morning and lifting weights. Probably, this person would not have the same performance as doing this task during the afternoon or in a more active period. Therefore, reversing the light-dark cycle of the animal´s room and train it during dark cycle (active period) could be an attractive choice. Many articles were published through this circadian adaptation [33, 34, 36, 37]. However, this theory is merely critical, and no study published to date has tested this hypothesis. A few suggestions might be made to test this theory such: measurement of circadian hormone levels, the number of climbs per training session, load parameters (absolute and relative training volume), stress markers and climbing pattern. The adaptation period to equipment is another variable that should be taken into consideration by researchers. This process is rarely discussed in the literature and indeed the animal would not climb the ladder of its free will and at the speed, we would like. Cassilhas et al. [38] used an adaptive protocol in which the animal performed three climbing attempts in three different ladder locations and during three consecutive days. In the first three attempts, the animal was placed near to the top of the equipment, next to the comfort area. On the second attempt trials, the animal was placed in the middle third of the material. And finally, on the third and final three attempts, each animal climbed the ladder from the lower base of the apparatus to the ladder´s top. The authors clocked the animal´s climbing time in the last trials of each day. The climbing time steadily decreased over the three days of adaptation. This result demonstrated clearly the animal learning process. However, other studies used different protocols and reported intriguing adaptation processes. Linderman et al. [39] acclimated the animals twice daily (5 climbs each time) for a week, using 20% of the animal body weight attached to the tail. Grossman et al. [40] decided to adapt the animals using three climbs for three times per day (morning, mid-day and mid-afternoon). Gradually, the authors raised the bound load on the animal's tail. They reported that it was able to climb the ladder with 70% of it body weight after only five days of adaptation. Clearly, these studies showed that there is no consensus about the best form to adapt it to the ladder equipment. Another question that we could ask is presented by the fact of: should we use or not the bounding load to animal´s tail during the process of adaptation? This discussion seems relevant because doing so; it could decrease the error of a mistaken measure of maximal strength on the maximal strength test session. This issue must possibly be verified by improvement of muscle coordination found in human training studies. Also, adaptation period with attached loads must increase training total volume, being so, critical to muscle hypertrophy and closely related to nearest start training quality. Finally, there were also other ways of load adjustment made through the typical reward models [12, 22]. Nevertheless, food or water deprivation might interfere directly with the psychological, physiological and morphological response to vertical training [8].
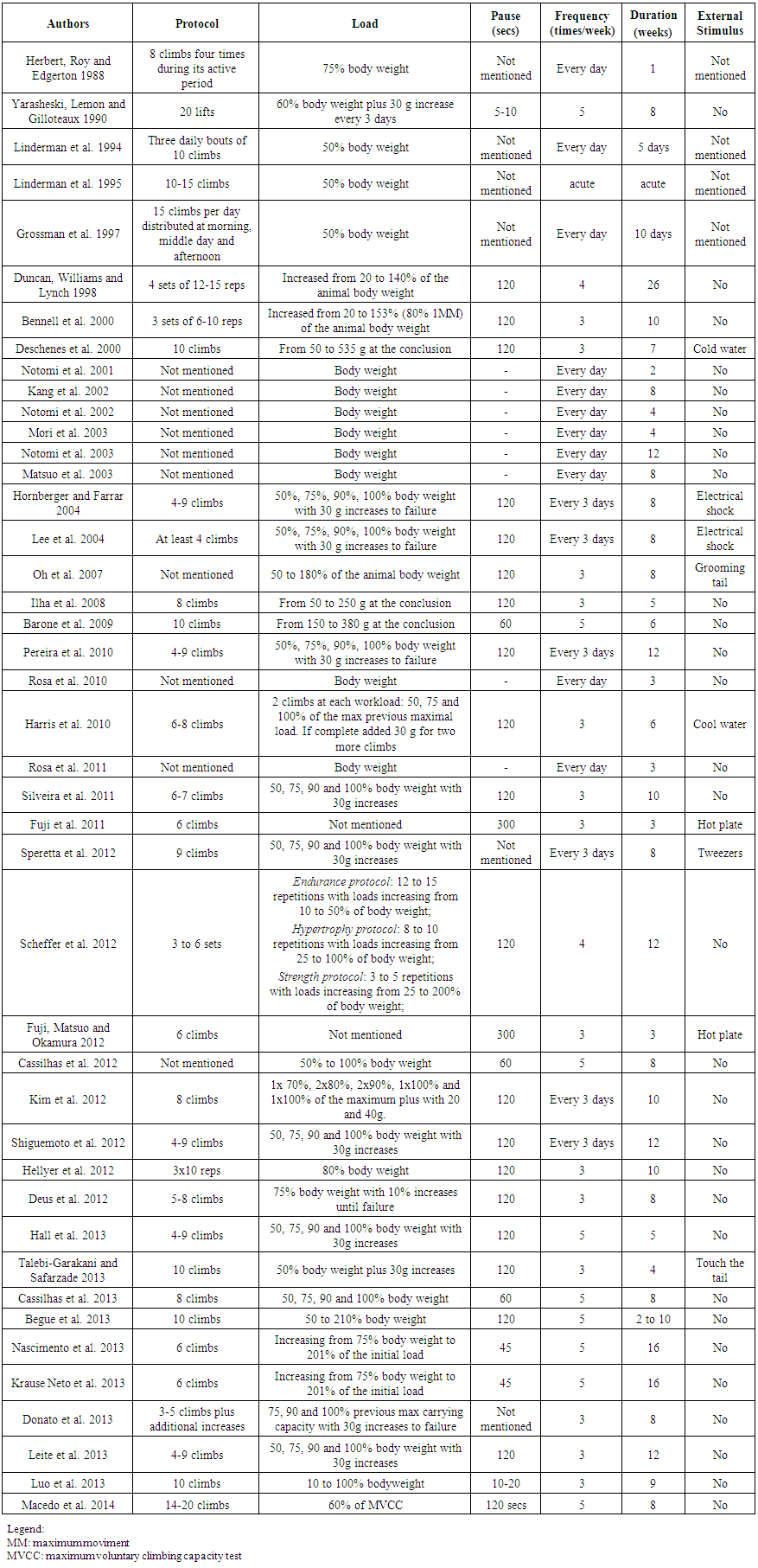 | Table 2. Description of the Resistance training Protocol Characteristics |
3.2. Maximum Carrying Load Test
- Using maximum load testing on studies involving human beings is commonplace. Many types of equipment are used for this purpose, and it range from free weight exercises with barbells to machines such as Isokinetic dynamometer. Recently, much discussion on the feasibility and the real need to apply maximum load tests in rodents are in progress. This discussion is getting serious attention, as it is impossible to know and be sure if the rodent performed maximum exertion during a standard load test. So far, a value for maximum strength or capacity would not get because it performs various dynamic movements up to the ladder´s top [6]. In this case, we might measure the maximum carrying load capacity, whose manifestation is the maximum resistance strength. Hornberger and Farrar [28] described the first attempt of making a full load testing. In their first training session, the rat climbed the ladder carrying up an equivalent load of 75% of it body weight. Once completed the climb, 30 grams were added to the burden bound in the animal's tail. This procedure was repeated until the animal could no longer climb the apparatus and achieve "failure." This term was determined when the animal could no longer climb the ladder for three consecutive attempts. A recent review suggested measuring venous lactate to adjust training loads appropriately [6]. Lactate is a salt derived from the reactions of the glycolytic metabolism, and it is considered a valid measurement of training intensity since it values increase in blood concomitantly with increased training load [41]. However, any external load attached to its tail must cause lactate increases just as stress result. Also, lactate measurement during training sessions might be different from maximum load testing sessions. Therefore, as far lactate must be taken, the need for a more accurately tool should be developed and studied. Recently, Deus et al. [41] described, which was called, Maximum resistance test (MRT). In this version, the animal climbed the ladder without additional burden to the body. From there, an increase of 10% of it body weight was performed every climb until the animal could no longer climb the ladder. At the beginning of the test and 60 seconds after each climbing, blood was withdrawn from the animal´s tail vein for plasma lactate measurement. Lactate analysis was done to demonstrate that animals were tested actually with high loads. This test was repeated before and after the experiment. In our view, the advantage of this type of blood testing is presented by the possibility of repeating it at the end of training period. It is trivial to compare the initial to the final training load results, through the maximum load achieved in the last training sessions and the comparison to the first loads. Harris et al. [42] used an initial amount of 50% of the animal body weight and progressed to failure as the protocol described by Lee et al. [13] and similar with Hornberger and Farrar [28]. In all cases, we could identify the maximum load carried by the end of training period. However, in human studies, testing are done in a pre to post manner. So, the best strategy to do is to conduct a test session before and another after the training period. Cholewa et al. [6] called this a “local muscular endurance test.” However, climbing is a multi-action movement. Thus, calling it “local” might not be the best description to do. Currently, is a consensus that the process of familiarization to maximum strength or strength endurance test should be respected. However, as previously mentioned, it did not clarify if before starting load testing, a familiarization process is required. The familiarization process aims to improve muscle coordination and lessen the effect of the first sessions of RT bias. Dias et al. [43] indicated the need for two or three sessions of familiarization testing for experienced men before using it as a parameter. For inexperienced individuals, Silva-Batista et al. [44] demonstrated that three or four familiarization sessions are needed. Thus, it is clear the need to investigate whether this phenomenon is also found in rodents. However, our hypotheses are that rodents might increase their ability to climb the ladder with higher loads and have faster strength increases, over the first four training sessions, such as humans beings. Finally, the proximity between humans and animal testing is controversial. To achieve maximum values, both need to be highly stimulated. Human studies protocol frequently used verbal screaming to encourage the volunteers during strength testing. Until now, only external tools, such as electrical shock, cold-water spray or tail grooming, were used to stimulate the rodents to ladder climbing [13, 31, 45]. Many animal care institutions complained about these techniques, arguing it might cause additional stress to animals. Perhaps, a less aggressive tool might also be developed.
3.3. Training Volume and Intensity
- Appropriate recovery between training sessions and a balance between volume and intensity are critical to improving the physiological adaptation to exercise. Currently, it is known that total volume of training (TVT) is essential to gain strength and muscle hypertrophy in humans [46-48]. Nevertheless, little is known about this training variable in animal training models. To calculate the TVT, for climbing training protocols is necessary to sum the load added to the animal's body weight over the number of climbing performed. So, if the rat climbs the ladder with 500 grams of the external load attached to its tail, over six times, then TVT would be 3 kg. However, if the added amount are progressively increased over climbs, we must need to calculate the average load carried during each climb. Therefore, we must sum the overall weight increased and divide it by the overall number of climbs. It apparently seems easy, however, an apparent difference between volume parameters were seen between animal climbing training and human strength training.The animal´s training volume must be calculated using the number of climbs. Of course, we would say that each climbing equals one repetition. However, it is worth remembering that the concept of repetition was established through a stretch-shortening cycle. According to Hornberger and Farrar [28], the animal performs an average of 8 to 12 dynamic movements per climb. That is, for each animal a total of 8 to 12 movement cycles or repetitions must be done during each climb. Thus, it should not be told that a climb equals one repetition. Also, there is a different and interesting way of getting TVT. By attachment of a recording camera to the side, or in front of the equipment, it is possible to ascertain how many moves the animal shall do from the bottom to the top of the ladder. This variable could be misunderstood; however, thinking that TVT is crucial for muscle gains, we suggested that it might be important to calculate the total volume per climbing (TVC). This new concept is calculated by multiplying the number of movements by the animal tied load in each climb. For example, if the animal climbed the ladder through nine moves tailed with 500 grams, so TVC of 4.5 kg was achieved at this climb. Thus, if the climbing pattern did not vary, for the same previous example, during six climbs the animal would do a TVT of 27 kg. This calculus must give us an idea of proper training adjustment. Perhaps, these simple methodological controls might bring near to a more appropriate and accurately load adaptation analysis, control, and progression. The manner in which the animal climbs, the ladder is also fundamental to reach a suitable morphological or physiological response. Cholewa et al. [6] hypothesize that multiple factors such as time of training and quality of the training load are necessary for gains in muscle hypertrophy. However, when it comes for rodents, the form of climbing also becomes another important variable. Intriguing is the fact that we cannot control the manner of which animal climbs the ladder. Again, filming it might help to control precisely training variable.Variables such as loading, climbing time and rest between each climb compose the intensity of training. Neither studies until today ever discussed; what is the best protocol for rodent vertical training? Therefore, several questions might rise such as: should training prescription be done by the percentage of it body weight or testing it for maximum carrying load capacity? These two conditions must still be discussed taking into consideration another factor: when should the load be increased; every session or every week of training? Hornberger and Farrar [28] prescribed the initial training load through a testing session done in the first training session. These authors used an initial loading of 75% of the animals' body weight to start. With the climbing success, increments of 30 grams were done until the animal reached the "failure". Failure was defined as the rodent impossibility of climbing the ladder for three consecutive attempts. After this initial session, the authors prescribed percentages of the previous maximum load at rates of 50%, 75%, 90% and 100% of the previous maximum voluntary load carried. After each success climb, again 30 grams were added until animal failure was reached again. Thus, the animal trained near to its maximum voluntary capacity at each training session. However, it is not certain if the animal got a failure. Recently, Harris et al. [42] adapted this protocol for older animals. The authors used a fixed number of climbs per training session. After identifying the maximum load of the animal in the first training session, the authors prescribed two climbs on each of the following amounts: 50, 75 and 100% of the previous maximum voluntary load carried. If the animal climbed the ladder six times, was added to it tail an extra 30 grams for one or two more attempts. Thus, reaching 100% max, a new load was adjusted again. Further, it is also possible to train the animal with the prescribed amount from it body weight. Thus, small progressive increases might be made just by weighting the animal every week. Also, the third option of loading prescription control stands to re-doing maximum load testing every week and prescribe training load using a percentage of it. Monitoring variance in training load and metabolic markers may be used and controlled. The work of Scheffer et al. [49] clearly demonstrated what we have been trying to describe and apparently discussed. The authors have divided animals into four different groups: Endurance Resistance Training (ERT), hypertrophy (HT), Strength (ST) and untrained group (UT). Training was applied by percentages of the animal´s body weight. Thus, training load for ERT, HT and ST groups were light (10%, 20%, 30%, 40% and 50%), intermediate (25%, 50%, 75% and 100%) and heavy (25% 50%, 100%, 125%, 150%, 175% and 200%), respectively. After that, each group performed a different number of repetitions (not clear if was a pause between it) [12-15 (ERT), 8-10 (HT) and 3-5 (ST)]. The number of sets (climbs) was maintained between 3 and 6 per session. The results demonstrated equally lactate levels pre to post training session between groups, and reported no significant difference in glycogen content between groups. Possibly, all groups presented similar results once TVT and TVC were not controlled. Moreover, authors did not publish the average load climbed (ALC) by each group. This variable might show the quality of the training session. ALC is calculated by the sum of every climb session load divided by the number of climbs performed. This variable showed to be an easy tool and allowed us precisely compare training evolution between many training groups (unpublished data). Clearly, there are several training prescription possibilities in the literature, however, was still not clear which is the best protocol available. Scheffer et al. [49] training protocol induced a great oxidative response. Cholewa et al. [6] suggested it to be, possibly, effective to muscular hypertrophy. Also, authors mentioned that more “sets” and less “repetitions” might be effective to induce muscular hypertrophy during ladder training. Perhaps, a more precisely volume control must show it. Human studies described that time under tension must be significant to get significant hypertrophic responses. After adaptation process, animals must climb the ladder voluntarily. Climbing might be faster in the beginning (external light load) and more slowly at the end of the incremental period ascertain session load and exhaustion might be at higher fatigue thresholds. Possibly, the addition of the climbing time measurement might be included to help explain some results. However, the measurement of climbing time is also rare. Among the many variables of training, the rest or pause between sets had possibly one of highest variability in human studies [50]. Apparently, resting time between climbs follows a clear consensus in animal research. 120 seconds pause between each climb is often used, and it shows few variations (Table 2). Shorter resting time might stimulate a higher resistance adjustment and subsequently muscle hypertrophy. But, at the lab, it sees that as the animal fatigue, it started to refuse to climb, even in the absence of attached load. This detail must be taken into consideration, and there is the need for further studies about it.
3.4. Duration
- The length of exercise training is always a matter of great debate. Here, we have demonstrated that a few training sessions can lead to positive results for some [39], while a longer intervention should be needed for others outcomes [51]. The duration of studies using ladder climbing might vary from one to 26 weeks. The week frequency must also be a key factor identifying a suitable closure. In Table 2, it is possible to overview that the week frequency might range from multiple daily sessions to more spaced ones (3x/week is the most common used). Thus, both the duration and frequency depended on the outcome to be studied. Herbert, Edgerton, and Roy [20] have shown that a week of training four times a day, every day, reduced the suspension-induced atrophy of rat limbs. Linderman et al. [52] demonstrated that a combination of Growth hormone and LC during five days attenuated the loss of myofibrillar content and synthesis in gastrocnemius muscle. Despite these positive short-term results, most studies demonstrated the need for more time for better results. Krause Neto et al. [33] and Nascimento et al. [34] trained middle aged rats for 16 weeks and demonstrated cross sectional area increases of Soleus, Extensor Digitorum Longus, and Triceps Brachialis muscles. However, Cassilhas et al. [38] showed cross sectional area increases of the Gastrocnemius and Flexor Digitorum Longus muscles in just eight weeks. Possibly, key cellular and molecular pathways in Ladder Climbing training studies might explain the reason for these variations.
4. Muscular Hypertrophy Mechanisms
- In recent years, many studies were conducted aiming to investigate the signaling pathways responsible for muscle hypertrophy process [53, 54]. Nevertheless, many studies have been carried out using models without physical effort [55-57]. Thus, it has become difficult and almost impossible to compare those results with vertical climbing studies. Also, many cellular and molecular mechanisms were investigated using a non-voluntarily device. This fact stand a factual question: Must we compare results from volunteer and non-volunteer studies or not? Thinking about this manner ahead follows a brief review of the cellular mechanism through vertical training studies. Yarasheski, Lemon and Gilloteaux [22] demonstrated that models of vertical climbing were adequate to stimulate muscle hypertrophy. However, Duncan, Williams and Lynch [23] failed to show this same phenomenon. The comparison between both works is difficult because of different protocols and skeletal muscles evaluation. Further, Hornberger and Farrar [28] described the Ladder climbing equipment and training protocol mostly used today. Their equipment enables easy construction and positive results to stimulate muscle hypertrophy of Flexor Hallucis Longus (FHL). Lee et al. [13] also showed that Ladder climbing increased muscle mass of FHL muscle in young adult rats. This effect was potentiated when combined with overexpression of IGF-1. Corroborating, Kraemer et al. [58] showed that RT induced dynamic adaptations in somatotroph structure and function. These facts might explain the supra effect of IGF in stimulating muscle hypertrophy since Growth hormone (Gh) and IGF-1 work together. Intriguing, Lee et al. [13] reported that LC equipment is modest in intensity when compared to other devices that used a larger number of repetitions and lower frequency of training sessions. Hellyer et al. [59] demonstrated that even moderate, Ladder climbing training was sufficient to increase the cross-sectional area (CSA) and content of myosin heavy chain (MyHC) in FHL muscle of young adolescent rats. However, this study demonstrated that even stimulating muscle hypertrophy, this type of training failed to increase the expression and phosphorylation of key factors (Akt/mTOR and rpS6) for muscle hypertrophy. The authors suggested that stimulatory mechanisms of muscle hypertrophy should be different between young and adults rats. Also, other important critical factors might be related to skeletal muscle hypertrophy. Begue et al. [60] investigated whether high intensity training could regulate the activity of the IL-6/STAT1/STAT3. Recent studies have shown that this cell signaling pathway is important for muscle hypertrophy [61, 62]. The authors compared the response of these molecules after 2, 4 and ten weeks of training in vertical climbing training. The rats achieved a loading of 210% of it body weight and 100% muscle hypertrophy after ten weeks of training. Moreover, acute measurements showed an increased expression of IL-6 mRNA concomitant with phosphorylation of STAT1 and STAT3 after 2 and 6 hours of training. A positive analysis of BrdU demonstrated increased satellite cells after training. Corroborating, Luo et al. [63] showed that Ladder climbing was effective to increase autophagy and reduce apoptosis of muscle cells through modulation of IGF-1 and its receptors and activation of Akt/mTOR, and Akt/FOXO3a signaling pathways in the muscle of old rats. Although this evidence demonstrated the activation of relevant cellular and molecular pathways that explained muscle hypertrophy seen in studies that used the Ladder climbing model, it is necessary to further studies.
5. Outcomes Studied beyond Muscular Hypertrophy
- Muscle hypertrophy is undoubtedly one of the most studied outcomes when dealing with experimental research involving vertical climbing training models [13, 22, 23, 28, 31, 38, 40, 59, 60, 64]. However, this equipment is also used in a broad range of other outcomes such as oxidative stress [49], changes in bone properties [65], insulin sensitivity and diabetes mellitus [9, 11], cancer [51], and many others [66-69]. Leite et al. [70] demonstrated that 12 weeks of training in Ladder climbing significantly increased Matrix metalloproteinase 2 (MMP-2) enzyme activity in left ventricle plus positive induced beneficial changes in body composition and reduced blood pressure of rats that received a high fat diet. Additionally, other studies also showed that LC caused an increase of MMP-2 in other structures such as the Achilles tendon and Tibia of ovariectomized rats [65, 71] and Biceps and Gastrocnemius muscles of diet-induced obese rats [72].We have already cited the difficulty of finding the best model for training rodents. The Ladder climbing has the advantage to recruit a lot of muscle mass, and it is known that muscle mobilization can positively affect the treatment of wide variety of metabolic disorders [9,10] and improve body composition (Souza et al. 2014). Talebi-Garakani and Safarzade [11] showed that four weeks of LC (3x/week) reduced the serum levels of Tumor necrosis factor (TNF-α), C-reactive protein (hs-CRP) and Interleukin (IL-6) in diabetic rats. Hall et al. [9] found an increased amount of glucose transporter (GLUT4) in skeletal muscles of rodents with type I diabetes after RT.The reduction in muscle mass due aging or as a side effect of some treatments must cause physical dependence. Thinking of minimizing this effect; the vertical climbing resistance training should be interesting and successful investment strategy for researchers of these areas. Harris et al. [42] found that six weeks of training (3x/week) improved endothelial dysfunction associated with aging. Cardiac and metabolic disorders might lead to increased apoptosis and muscle atrophy. Luo et al. [63] suggested that RT is associated with increased autophagic activity and reduced muscle apoptosis. This phenomenon could be explained by Insulin like growth factor (IGF-1) and its receptors action, and cellular signaling mechanisms like Akt/mTOR and Akt/FOXO3 in aged muscles [63]. Routinely, muscle atrophy was seen as a side effect of limb immobilization. Studies showed that only a few sessions of Ladder climbing might attenuate limb muscle atrophy [20, 39, 52]. However, it seems that RT may not be as effective as aerobic training in stimulating peripheral nerve regeneration in rats [73]. The application of glucocorticoids can interfere with muscle responses to RT. Thus, Macedo et al. [74] found that eight weeks of Ladder training with low intensity is sufficient to attenuate the processes of protein degradation induced by dexamethasone treatment. Finally, Donato et al. [51] showed a powerful effect of Ladder Climbing in preventing symptoms of cancer-induced cachexia.
6. Conclusions
- This review raised factual questions about the prescription of resistance training using the vertical climbing model for rodents. Training for rodents is unique and there are many differences when compared to human training. These differences have been demonstrated both in the analysis of the type of exercise and the training protocols. Variables such as volume and intensity shall be described and control in future articles using TVT, TVC, ALC calculus, climbing and resting time. Climbing biomechanics and maximum voluntary load testing shall be done and described. Researches should control all variables to ensure that result suffered the lowered bias possible. For last, LC equipment demonstrated to be useful in studying a great diversity of outcomes.
ACKNOWLEDGEMENTS
- We would like to acknowledge Professor Danilo Bocalini for his support and time spent to discuss critical features of this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML