-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Sports Science
p-ISSN: 2169-8759 e-ISSN: 2169-8791
2016; 6(1A): 12-18
doi:10.5923/s.sports.201601.03

Heart Rate Profile and Variability in Cross Country Cycling Athletes
Eurico Ribeiro1, C. A. Fontes Ribeiro2, Paula Tavares1, 2
1Laboratory of Muscle Physiology, Faculty of Sport Sciences and Physical Education, University of Coimbra, Portugal
2Department of Pharmacology and Therapeutics, Faculty of Medicine, Portugal
Correspondence to: Paula Tavares, Laboratory of Muscle Physiology, Faculty of Sport Sciences and Physical Education, University of Coimbra, Portugal.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Cross-country cycling is a very demanding sport. The characteristics of the competition are mainly aerobic but in a maximal effort. Due to the heart rat achieved during competition we may expect that there is some cardiovascular risk involved. Thus, the main aim of this work was to study the autonomic nervous system balance and heart rate recovering in cross-country cycling athletes. The autonomic nervous system was evaluated by measuring the heart rate variability. Our results suggested that in those athletes there is a very good balance between sympathetic and parasympathetic systems as well as a very efficient heart rate recovery. So, there is no indication of cardiovascular risk, increase in mortality or sudden death.
Keywords: Cross-Country Cycling, Heart Rate Variability, Autonomic Nervous System, Cardiovascular Risk
Cite this paper: Eurico Ribeiro, C. A. Fontes Ribeiro, Paula Tavares, Heart Rate Profile and Variability in Cross Country Cycling Athletes, International Journal of Sports Science, Vol. 6 No. 1A, 2016, pp. 12-18. doi: 10.5923/s.sports.201601.03.
Article Outline
1. Introduction
- Cross-Country cycling is an endurance competition starting with pedestrian control, involving several turns in order to complete a mountain trail, normally 6-9 km long, and an average accumulative altitude of ascents and descents of about 1500 m. UCI (International Cycling Union) suggests a different number of turns and time racing around 120-135 minutes for men and 105-120 minutes for women. The starting grid is set according to the UCI points system (for international events) and/or national points system for national races. This is a popular outdoor recreational activity and an Olympic Sport [1]. Cross-country circuit races are performed at an average heart rate (HR) close to 90% of the maximum heart rate, and more than 80% of race time is spent above the lactate threshold [1, 2]. The high exercise intensity is related to the fast starting phase of the race, several climbs, greater rolling resistance, and the isometric contractions of arm and leg muscles necessary for bike handling and stabilisation. Due to the high power output (up to 500W) required during steep climbing and the start of the race, anaerobic energy metabolism plays a key role on off-road cycling [1]. Mountain biker’s physiological characteristics indicate that aerobic power (VO2max >70 mL/kg/min) and the ability to sustain high work rates for prolonged periods of time are prerequisites for competing at a high level in off-road cycling events. Cross-country is also characterised by a great diversity of terrains and trails, where single tracks are technically very difficult. This amount of characteristics and conditions leads to a permanently elevated HR [3], and like other aerobic sports excels in high physical demand, especially at cardiovascular level. So far, it is unknown whether the correlation between the recurrent practice of this modality in intensities and possible risk for the cardiovascular system, knowing that extreme mountain bike challenges may induce sub-clinical myocardial damage [4]. Although it’s known that the practice of mountain biking in submaximal intensities has beneficial effects on amateur riders [5], monitoring of exercise performance and intensity during competition presents several limitations. In this regard, field data collection requires special technology for the evaluation and storage of the information with accuracy for a long duration, and occasionally, under adverse situations. The most accessible and primary variable monitored parameter is the HR, which can be used to establish the competitive event intensity profile, to analyse competitive stress and training responses, and to detect early overtraining [6]. While substantial numbers of scientific papers have been published to describe the exercise intensity based on the HR data during road cycling, there are few scientific papers focusing the off-road cycling. Little, however, is known regarding the physiological demands of mountain-bike competition [7].Several parameters may be used to evaluate cardiovascular risk in a non-invasive way. Among them, there is the recovery heart rate one minute after effort (recovery) and the balance between the sympathetic and the parasympathetic nervous systems by the analysis of heart rate variability. These method enables observation of the fluctuations during short to long periods of time, is a non-invasive and selective observation of autonomic function. The heart rate variability has been used to study the autonomic nervous system under various conditions such as physiological, psychological and pathological [8]. The clinical usefulness of heart rate variability to identify changes in the autonomic nervous system has been enhanced through different methods [9]. The heart rate variability can be calculated by the mathematical equation in the electrocardiogram (ECG) or by changes of R-R intervals analysing their different domains and nonlinear geometry, which reflects the differences in the sympathetic and parasympathetic nervous system modulation. In this way and using the analysis of heart rate variability, we intend to assess autonomic function modulation as one of the predictors of inherent cardiovascular risks for Cross-Country cycling, since few studies have investigated the physiological parameters of these athletes [10]. Most of them only report that the Cross Country racing circuit is performed at maximal and submaximal intensities, with average heart rate close to 90% of maximum heart rate, where also can exceed 2 hours long [2]. According to the described, the risk/benefit of Cross-Country cycling is unknown. The major attention goes to intermittent and maximum anaerobic periods that may result in cardiac heart failure and/or autonomic dysfunctions [1].Taking into account the previously described, the main purpose of the present study was to assess the heart rate variability dynamics, especially autonomic function, and the evaluation of cardiovascular risk using resting heart rate. We also wanted to analyse the heart rate recovery at one minute after the exercise, as it’s been demonstrated the relationship between autonomic function and risk of mortality and sudden death [15].
2. Methodology
2.1. Experimental Design
- The experimental design was performed in 3 stages. We first assessed the anthropometric components, body composition and basal heart rate.Following and in a different day, athletes performed a maximal field test on a hardtaill mountain bike (only front suspension with 26’’ wheel) in a cross country circuit. We evaluated the heart rate variability during effort and cardiac recovery after the exercise.The last component of the study includes the assessment of heart rate and heart rate variability with software and specific tools.
2.2. Participants and Procedure
- Ten healthy male cyclist athletes (10 men) participated in the study. All subjects were non-smokers and free of known cardiac, respiratory or other diseases. The team was of Under 23 (6 athletes) and Elite (4 athletes) echelon, recruited at Portuguese Cycling Federation. It has been given a written informed consented to participate, and also transmitted the right to withdraw from the study at any time. At this moment a formulary was provided, where the athletes would have to insert the 3 days basal heart rate assessed on waking intervals. All of them didn’t use tobacco and/or drugs that could interfere with the cardiovascular variables that were being measured in this study. Additionally, the subjects didn’t ingest alcohol and/or caffeinated substances for at least 12h before the exercise session and neither participated in physical exercise and/or vigorous activity for at least 24h before the experimental session.
2.3. Heart Rate Variability Measurements
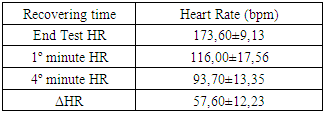
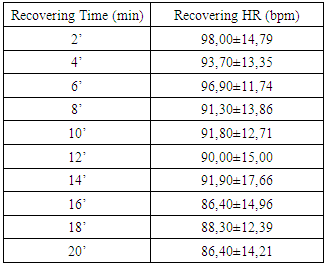
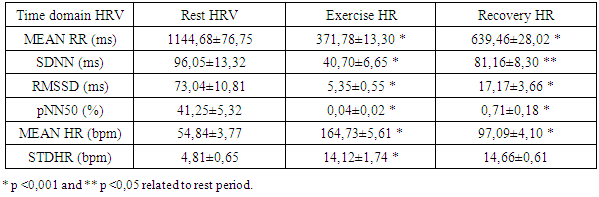
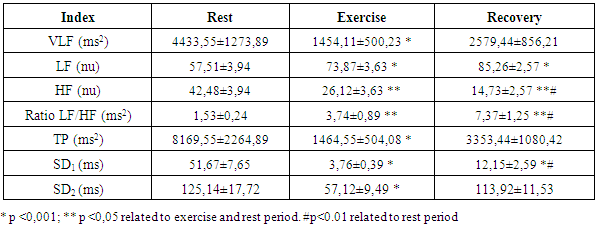
- Each HRV index was analysed during the experience and after the recovery, getting performed a global average value to each one. Beat-to-beat HR was also analysed during the 20 min recovery passive period immediately after the exercise. As soon as the exercise stopped, all subjects immediately stayed in supine position adjacent to the track. The interval of time between the end of exercise and the decubitus dorsal position was just 5 s. Particular attention to this detail has been taken because different body stances result in different absolute values for heart rate recover (HRR). The first HRR index was defined as the absolute difference between the final HR observed at the end of the exercise and the HR recorded 60 s after [11]. The second HRR index was calculated after the 4th min of the end of the exercise, and the third index was valued 20 minutes after the exercise. After an application of conductive gel, an electrode transmitter belt (T61, Polar Electro, Kempele, Finland) was fitted to the chest of each subject as instructed by the manufacturer. A Polar Electro S810 HR monitor was used to continuously record beat-to-beat HR during the exercise and subsequent recovery phase. All R-R series recorded by the S810 were extracted on a compatible personal computer with the processing software program Polar Pro Trainer 5 (v.5.40.172, Polar Electro). Occasional ectopic beats (irregularity of the heart rhythm involving extra or skipped heartbeats (i.e., extrasistole and consecutive compensatory pause) were identified and replaced with interpolated adjacent R-R interval values. We choose not to control the respiratory rate because the measurements were implemented in the field and because of that we didn´t want to interfere on natural return of HR to the baseline. The HRV parameters of the variables that were related to time, frequency and nonlinear domains were performed with the Kubius HRV software (v2.0, 2008 Finland). To smooth out transient outliers in the HRV plots (HRR vs. time in recovery), a median filter operation was introduced, so that, each value was replaced by the median of the value as well as the preceding and following values [11].Concerning HRV parameters, they were analysed in the time domain by the values of MEANRR (average of all normal R-R wave intervals), STDRR (standard deviation of all normal R wave to R wave intervals), RMSSD (square root mean squares of the successive sum of R-R interval differences), pNN50 (number of successive R-R intervals differing by 50 ms divided by the total number of successive R-R intervals). The HRV was also analysed in the frequency domain by the values of VLF (very low frequency range), LFnu (power in low frequency range), HFnu (power in high frequency range with normalized unit), by the ratio LF/HF (low frequency to high frequency), and also by the analysis of TP (variance of all R-R intervals). We also recorded the MEANHR-heart rate average and STDHR-standard deviation of heart rate average. We decided to normalize LF and HF to reduce the effects of noise due to artefacts and to minimize the effects of the changes in total power on the LF and HF components [12]. After this, the nonlinear indexes SD1 (represents the dispersion of points perpendicular to the line of identity) and SD2 (represents the dispersion of points along the line of identity) were also calculated.
2.4. Body Composition Assessment
- Initially, each subject’s body mass was measured using a digital scale (Seca 770), and his height was measure using a wooden stadiometer (Holtain Limited 98.603). Then, the hip and waist perimeters of each athlete were measured with a 1.5m flexible tape (Holtain). After this, we evaluated the body composition using surface bio impedance Soft Tissue Analyser - STA Bia 101. With the athlete in decubitus dorsal position and upper and lower members in 45° abduction, it was necessary to clean the skin with alcohol and cotton wool, followed by the placement of electrodes in the third metacarpal bone of the middle finger (negative electrode) and medial area of carpal bones (positive electrode). At the foot, electrodes were placed in the third metatarsal bone of the middle finger (negative electrode) and in the intermediate position of the hock joint (positive electrode). The final result was obtained by averaging three evaluations of bioimpedance analyser.
2.5. Field Protocol
- Before the beginning of the test, subjects were familiarized with the cross country track and conditions. Next, all parameters relative to heart rate and heart rate variability were assessed for each participant using a hardtaill mountain bike, and a cross-country trail with 6,2 km. This had the same length and characteristics of Cross-Country World Cup stage (trail delimited and closed to traffic), and it was transmitted to each athlete to perform the lap in maximum intensity in order to achieve the maximum heart rate. For this, each athlete was free to choose the gear relations during the test, like also the different positions on the bike (sited, up on pedals). All of athlete’s tests were performed always at the same time of the day, so that to be possible to respect circadian rhythms. At the end of each one, we proceeded to monitoring heart rate recovery and heart rate variability 1 and 20 minutes post-exercise, with all athletes in supine position. The heart rate evaluation was performed with a cardiofrequencimeter Polar S810 with beat by beat record during entirely field test and during recovery phase.
2.6. Statistical Analysis
- To characterize the sample and the variables in the different domains descriptive statistics were used, using the mean as a measure of central tendency, and the standard deviation and error as measures of dispersion. To characterize anthropometric, heart rate and body composition assessments, we used mean and standard deviation, and to analyse HRV components, we use mean and standard error. In inferential analysis and after tested normal distribution with nonparametric Kolmogorov- Smirnov Z, we used the Mann Whitney U test to compare results of independent samples. Differences between means were considered significant when p≤0,05 (significance level of 95%), and all of the calculations realized using SPSS v17.0 software.
3. Results
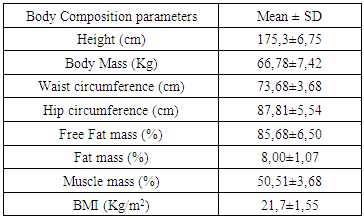
- The goal of the present study was to evaluate the cardiovascular risk in Cross Country cycling by the analysis of heart rate recovery and the autonomic function. The autonomic function was assessed by the analysis of heart rate variability in time domain (MEANRR, STDRR, RMSSD, pNN50, MEANHR and STDHR), frequency domain (VLF, LFnu, HFnu, TP and LF/HF) and geometric (SD1 and SD2). The body composition, such as obesity indicators, like the ratio waist/hip, was also evaluated.Concerning body composition our results shown a reduced percentage of fat mass in the present age interval. Besides, when compared to the general population, these athletes showed a decrease of the body mass index (table 1).
|
|
|
|
|
4. Discussion
- There is a lack of information concerning physiological characterization and cardiovascular risk of a so physical demanding sport such as Cross-Country cycling. The hard conditions of the competition lead to suppose that some cardiovascular risk was involved as has been described previously by Ortega studies [4].There are several indicators to assess cardiovascular risk such as body composition (namely waist and hip circumferences), heart rate variation (rest values and the decay at the first minute of recovery) and the function of the autonomic nervous system (ANS). Regarding body composition these athletes presented values that don’t contribute for cardiovascular risk, as it was expected. Thus, most important that BMI, the hip and waist perimeters are in the normal values indicated a reduced abdominal fat. Also, the composition of free fat mass and muscle mass are in agreement with their athlete condition.Baseline and rest heart rate are also parameters that must be considered when we study the cardiovascular system. In the present work the results showed very low values, which brings benefits to the athlete’s heart. However, since 2005 that a work of Jouven calls our attention to the risk of mortality and sudden death in athletes based on rest HR and HR values after exercise. To achieve that probability the authors consider the difference between the maximal heart rate and the heart rate after one minute of recovery [15]. Thus, at passive recovery less than 25 bpm (after the termination of the exercise) indicate a high risk for mortality and sudden death. In the present work we verify that our athletes have a rest HR less than 75 bpm and a recovery HR higher than 25 bpm (57,60 ± 12,23 bpm).The good HR behaviour must be related to a good balance in the ANS. As we previously described, the most practical and, nevertheless accurate, method to evaluate the ANS is the measurement of HRV, with some correlation, also, with pathological stats [16]. The HRV can be analysed through several indexes in the frequency and time domain giving information about the sympathetic and parasympathetic nervous system status.Analysis of the spectral density correspond to the frequency domain, which examines how the power (variance) is distributed as a function of frequency, and time domain variability can be measured by the statistical method or the geometrical, selecting each method correspondent to the particular objective in each case [17].Independent calculation of power spectral density (Fast Fourier Transform or autoregressive model) bounding up normally three distinct frequency bands called spectral components, and 3 types can be identified. High frequency (0.15 to 0.40 Hz) is modulated by the parasympathetic nervous system and by ventilation, while low frequency (0.04 to 0.15 Hz) and very low frequency (0.01 to 0.04 Hz) are modulated by both the sympathetic nervous system and the parasympathetic nervous system [8], although low frequency reflects predominantly the Sympathetic modulation.Several physiological correlations are studied on the analysis of spectral components. The high frequency spectral components are achieved only through the vagus nerve or parasympathetic nervous system, whereas, the potency of very low frequency is a result of the modulation performed by the sympathetic and parasympathetic nervous system influenced partly by other neurohumoral activities (for example, the renin-angiotensin-aldosterone system [18]. The potential benefits on analysis of frequency domain in heart rate variability is the ability to identify oscillations in frequency in heart rate signals that may be interpreted as different physiological mechanisms and thereby identify a neurocardiac regulation. Still, the high-frequency peak around 0.15-0.4 Hz corresponds to a respiratory sinus arrhythmia, mediated by the action of the parasympathetic nervous system and the low-frequency peak, ranging between 0.04 and 0.15Hz, is the result of the action of the sympathetic modulation [18]. Time domain analysis is another method of assessing cardiovascular sequences of oscillations, obtained from the calculation of the dispersion around the mean heart rate over a prolonged period analysed.The simplest method for evaluating the heart rate variability is the measurement in time domain, where heart rate is determined at any point in time or in the corresponding R-R intervals. Since it is non-invasive, this method has been shown to have important clinical utility for assessing the integrity of neurocardiac system and to identify the relative importance of regulating sympathetic and parasympathetic in the diagnosis of heart disease and autonomic nervous system [19].Studies comprising heart rate variability in progressive physical efforts suggest that parasympathetic modulation tends to decrease gradually until their full withdrawal when reached approximately 60% of VO2max. It’s suggested that the increment in exercise intensity corresponds to terminus of vagal influence and a most significant involvement of the sympathetic modulation, which can be related with the anaerobic threshold. The lactate threshold was compared to the intensity of physical effort in which, each athlete had a reduced vagal modulation, calling itself heart rate variability threshold [20, 1]. There was a coincidence between the lactate threshold and the heart rate variability threshold, making evident a possible cause/effect relationship between autonomic and metabolic events [20]. The return to resting heart rate levels after physical exercise it depends on the interaction of the autonomic functions, the fitness level and the workout intensity. The recovery can take from 1 hour after a mild or moderate workout to 4 hours after long duration aerobic exercise and up to 24 hours after intense exercise or maximum levels. The mechanism responsible for such differences in time recovery is not clear but some studies indicate that the most plausible explanation is the increase in vagal activity and decrease in sympathetic dominance [21]. Recent studies reveal that frequent practice of light physical exercise by women mid 50 years with controlled heart rate between 100 and 120 beats per minute (bpm), performing three times a week during two years is responsible for the improvement on cardiac autonomic function. Analysing the heart rate variability, it was possible to identify a significant increase in vagal and sympathetic modulation (frequency domain). Similarly, in the time domain, the analysis of heart rate variability demonstrate significant improvements on the components that reflect the vagal activity on active women, being considered extremely useful on health prevention and heart protection [22].In our study we observed a very good function of ANS in all the components of the HRV that we analysed.
5. Conclusions
- As it is well known, several factors may contribute to cardiovascular risk. In non-athletes the body composition, namely the increase in fat mass is one of the primary indexes. In athletes the body composition is also important with special relevance to waist circumference and its ratio with the hip size (waist/hip ratio). Beside these important parameters, the cardiovascular function after adaptation to sport effort is of major importance to cardiovascular risk. Thus, in this work we found a normal body composition in all athletes.The evaluation of cardiovascular risk in off road cycling was fundamental and urgent, taking into account not only the number of emerging practitioners, but also because of the scarcity of information on the topic.The analysis of heart rate variability and its variation, allows depict the functioning of the autonomic nervous system in a non-invasive, rapid and easy accessibility.We analysed the variation in heart rate after exercise and also an obesity indicator comparing waist/hip circumference. The change in heart rate after exercise caused a marked decrease of the same with 57.60 ± 12.23 beats per minute. The study revealed heart rate recoveries considered far superior to cardiovascular risk, as the American College of Sports Medicine establishes the absence of cardiovascular risk in cardiac recoveries above 25 beats per minute (passive recovery). The present results suggest that there is a reduced risk of mortality and sudden death in the presented athletes. The body mass index of the sample also shows no cardiovascular risk factor, together with the obesity indicator of compared waist/hip circumference lower than stipulated as cardiovascular risk. Resting heart rate was also lower than the average population confirmed with the reduced average resting heart rate, with approximately 51 beats per minute.In the present study the main goal was to assess the autonomic function and a possible cardiovascular risk in off road cycling through analyses of the heart rate variability and heart rate recovery. Based on this, we may suggest that after the end of the exercises, it occurs a rapid parasympathetic reactivation causing a rapidly heart rate decreases lowering, this way, the risk of mortality and sudden death. It was possible to observe a balance between sympathetic and vagal function during different times of analysis, suggesting a high autonomic modelling derived from practise of off road cycling. Based in SDNN, we affirm that this remains elevated during moderate intensity exercise, experiencing an increase in the transition of the exercise period of maximum intensity for the recovery/rest period.
ACKNOWLEDGEMENTS
- The authors want to express their thanks to Drª Fátima Rosado for the technical support in the laboratory. The authors wish to express their gratitude to all the athletes that participate in this study.
References
| [1] | Impellizzeri F M, Marcora S M. The Physiology of mountain biking. Sports Med. 2007; 37 (1): 59 -71. |
| [2] | Stapelfeldt, B, Schwirtz, A, Schumacher, YO, Hillebrecht, M. Workload demands in mountain bike racing. Int J Sports Med. 2004; 24 (4): 294-300. |
| [3] | Impellizzeri F M, Marcora S M, Rampinini E, Mognoni P, Sassi A - Correlations between physiological variables and performance in high level cross country off road cyclists. Br J Sports Med 2005; 39:747-751. |
| [4] | Ortega FB, Ruiz JR, Gutiérrez A, Castillo MJ. 2006. The Journal of Sports Medicine and Physical Fitness [2006, 46(3):489-493]. The Journal of Sports Medicine and Physical Fitness. 46(3):489-493. |
| [5] | Leicht, A. Allen, G. Hoey, A. 2003. Influence of Intensive Cycling Training on Heart Rate Variability during Rest and Exercise. Canadian Journal of Applied Physiology. 28(6): 898-909. |
| [6] | Costa, O. Oliveira, F. 2010. A resposta de frequência cardíaca durante as competições de “mountain bike cross-country”. Rev. Bras. Educ. Fís. Esporte. 24(3), 379-87. |
| [7] | Carpes, FP. Mota, CB. Faria, I. 2007. Heart rate response during a mountain bike event: a case report. Journal of Exercise Physiology online. 10(1), February. |
| [8] | Roberts W., 2009. Heart rate variability with deep breathing as a clinical test of cardio vagal function. Cleveland Clinic Journal of Medicine. 76 (Suppl 2), 37-S40. |
| [9] | Ribeiro P., Polanczyk A., Rohde P., Moraes S., Leite C., 1998. Sympathetic nervous system representation in time and frequency domain indices of heart rate variability. J Appl Physiol. 79: 69-73. |
| [10] | Impellizzeri F, Sassi A, Rodriguez-Alonso M, Mognoni P, Marcora S. Exercise intensity during off-road cycling competitions. Med Sci Sports Exerc. 2002; 34(11): 1808-1813. |
| [11] | Buchheit, M. Laursen, P. Ahmaidi, S. 2007. Parasympathetic reactivation after repeated sprint exercise. Am J Physiol Heart Circ Physiol. 293: 133–141. |
| [12] | Sztajzel, J. 2004. Heart rate variability: a non-invasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly. 134:514–522. |
| [13] | Novais LD, Sakabe DI, Takahashi ACM, Gongora H, Taciro C., Martins LEB. Avaliação da variabilidade da frequência cardíaca em repouso de homens saudáveis sedentários e de hipertensos e coronariopatias em treinamento físico. Rev Bras Fisioter. 2004; 8 (3): 207-213. |
| [14] | Godoy MF, Takakura IT, Correa PR. Relevância da análise do comportamento dinâmico não-linear (Teoria do Caos) como elemento prognóstico de morbilidade e mortalidade em pacientes submetidos à cirurgia de revascularização miocárdica. Arq Cienc Saúde. 2005; 12 (4):167-171. |
| [15] | Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon M, Ducimetière P. Heart-Rate Profile during Exercise as a Predictor of Sudden Death. N Engl J Med 2005; 352:1951-1958. |
| [16] | Routledg FS, Campbell TS, McFretridge-Durdle JA, Bacon SL. Improvement in heart rate variability with exercise theraphy. Can J Cardiol. 2010; 26 (6): 303-312. |
| [17] | Task Force of the European Society of Cardiology the North American Society of Pacing Electrophisiology. Standards of Measurement, Physiological Interpretation, and Clinical Use. 1996. 93 (5):1043-1065, Londres. |
| [18] | Takase B., Kitamura H., Noritake M., Nagase T., Kurita A., Ohsuzu F., Mathuoka T., 2002. Assessment of Diabetic Autonomic Neuropathy using Twenty-Four-Hour Spectral Analysis of Heart Rate Variability. Japanese Heart Journal. 43 (2). |
| [19] | Ribeiro F., Cunha A., Lourenço D., Marães S., Catai M., Gallo L., Silva E., 2000. Estudo da variabilidade da frequência Cardíaca em dois voluntários de meia-idade, um coronariopata e outro saudável - relato de caso”, Rev. Soc. Cardiologia Estado de São Paulo. 10 (1) (Supl 1). |
| [20] | Brunetto, A.. Roseguini, B. Silva, B. Hirai, D. Ronque, E. Guedes, D. 2008. Limiar de variabilidade da frequência cardíaca em adolescentes obesos e não-obesos. Revista Brasileira de Medicina do Desporto. 14 (2). |
| [21] | Alemida M., Araújo C., 2003. Effects of aerobic training on heart rate. Revista Brasileira de Medicina do Desporto. 9 (2). |
| [22] | Paschoal A., Polessi E., Simioni F., 2008. Avaliação da variabilidade da frequência cardíaca em mulheres climatéricas treinadas e sedentárias. Arquivo Brasileiro de Cardiologia. 90 (2). |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML