-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Sports Science
p-ISSN: 2169-8759 e-ISSN: 2169-8791
2014; 4(6A): 12-18
doi:10.5923/s.sports.201401.02
Passive, Active, and Cryotherapy Post-Match Recovery Strategies Induce Similar Immunological Response in Soccer Players
Jader de Andrade Bezerra1, Antônio Clodoaldo Melo de Castro1, Sandro Victor Alves Melo1, Faber Sérgio Bastos Martins2, Romeu Paulo Martins Silva1, José Augusto Rodrigues dos Santos3
1Federal University of Acre, Brazil
2School of Education of Fafe, Portugal
3Faculty of Sports, University of Porto, Portugal
Correspondence to: Jader de Andrade Bezerra, Federal University of Acre, Brazil.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Recovery strategies in soccer are key for restoration of the metabolically challenged body homeostasis. To better understand the mechanisms of recovery, we analyzed the hematologic and immunological responses of three different recovery strategies after competitive soccer match play. Forty-two male professional soccer players (age, 25.7 ± 4.6 years; body mass, 75.8 ± 6.4 kg; % body fat, 11.0 ± 2.2%; VO2max, 50.4 ± 3.9 mL/kg/min) were followed-up during three consecutive days. The players were divided into three equal groups (n = 14), each of which performed one of three recovery programs after completing a soccer match: passive recovery (PR), active/jogging recovery (AR), and cryotherapy (CR). Blood samples were collected before, immediately after, and 24, 48, and 72 hrs after the match. After the match, the hematologic parameters were not significantly different between groups (p > 0.05). Regardless of the recovery strategy, significant changes were observed after the match in erythrocyte count (hemoglobin and hematocrit), yet with no physiological value. All groups showed post-match leukocytosis, which was mostly a reflection of increased neutrophil and monocyte count (p < 0.05). Match-induced leukocytosis was reversed during the recovery period. The metabolic demands of a soccer game were not sufficient to elicit a hematologic response with physiological meaning. Passive, active, and cryotherapy recovery strategies similarly reversed match-induced immunological responses in soccer players.
Keywords: Cold immersion, Football, Jogging, Recovery training
Cite this paper: Jader de Andrade Bezerra, Antônio Clodoaldo Melo de Castro, Sandro Victor Alves Melo, Faber Sérgio Bastos Martins, Romeu Paulo Martins Silva, José Augusto Rodrigues dos Santos, Passive, Active, and Cryotherapy Post-Match Recovery Strategies Induce Similar Immunological Response in Soccer Players, International Journal of Sports Science, Vol. 4 No. 6A, 2014, pp. 12-18. doi: 10.5923/s.sports.201401.02.
Article Outline
1. Introduction
- Soccer is a complex and demanding sport that combines cyclic and acyclic movements with high eccentric loading [1]. Recent studies [2–7] have demonstrated that eccentric contractions have a considerable impact on the hematologic response (hemolysis) and on several immunological markers (leukocytosis, neutrophilia).From a metabolic perspective, soccer is essentially an aerobic sport [8] with sporadic outbursts of high-intensity sprinting, which tax lactic and alactic anaerobic systems. Despite its highly aerobic nature, soccer is very demanding metabolically, and significantly disrupts tissue homeostasis, especially in skeletal muscle [9].The stress induced by long and intense exercise might trigger a series of acute hematologic and immunological changes (hemogram, leukogram). The time of recovery to basal values depends on the duration, intensity, and type of exercise. For instance, the extent of changes in total leukocyte and neutrophil count depends on exercise intensity [10].Sometimes during competition season, the recovery time between two matches can be as short as 72 hours, and return to training sessions can occur within the first 24 hours after a match. However, such short times might be insufficient for normalization of the physical performance of the athletes [11]. Therefore, recovery training has become increasingly important, and is now recognized as key in the development of performance and injury prevention [12, 13].A variety of different recovery training methods are incorporated into training design to help in recovery and fast return to play [14, 15]. Active recovery with low-intensity exercise provides good recovery [16, 17], especially favoring the clearance of blood lactate [18, 19] and creatine kinase [20]. Cryotherapy, another method for reducing the symptoms of delayed-onset muscle soreness, is still matter of controversy [21, 22]: some studies have reported good results [23-25], while others failed to find significant improvements in a variety of parameters [13, 26, 27].The recovery process directly influences the ability to return to normal training and performance levels. In soccer, the mechanisms of recovery have not yet been fully investigated. To add information about the current recovery methods in soccer, we investigated how and to what extent do three different recovery strategies—passive recovery, active recovery, and cryotherapy—affect several hematologic and immunological biomarkers after a soccer match.
2. Methods
2.1. Participants
- Participants were 42 male professional soccer players (age, 25.7 ± 4.6 years) who played in first division soccer championship in 2012. Prior to the study, all participants were informed of the nature of the experimental procedures and gave their written consent. The study was approved by the ethical review board of Fundação Hospitalar do Acre (FUNDACRE) (approval no. 648/2011).
2.2. Experimental Design and Procedures
- The study was conducted during seven matches of the 2012 Acre Championship (Acre, Brazil). Each subjected was evaluated at a single match. Seventy-two hrs before each match, selected players were subjected to anthropometric measurements and to shuttle run test [28] for an estimation of maximal oxygen consumption (VO2max). Five to seven athletes were selected per match. On the day of each match, the players were asked to arrive at the venue 2 hrs before the start of the match. During this 2-hr period, the first blood samples were withdrawn. Blood collection was also performed immediately after the match in players who completed the full 90 min of the match and suffered no injuries. Blood was also collected at 24, 48, and 72 hrs after each match. The players were not allowed to consume any dietetic supplements or ergogenic aids before, during and for a few days after the match. Water was made available ad libitum during and after each match. During the three-day recovery period, all players maintained the same diet as provided by their own club.The recovery protocols were administered after the immediate post-match blood sampling. The participants were randomly divided into three groups of 14 players. Recovery protocols were administered after competitive match play: the passive recovery group (PR) did not perform any training during the three days following the match; the active recovery group (AR) performed three 30-min training sessions (one training session per day) consisting of continuous jogging at 50% of the individual’s VO2max; and the cryotherapy group (CR) performed three 10-min sessions (one session per day) of immersion to iliac crest level into stirred cold water (10°C).
2.3. Hematologic and Immunological Analysis
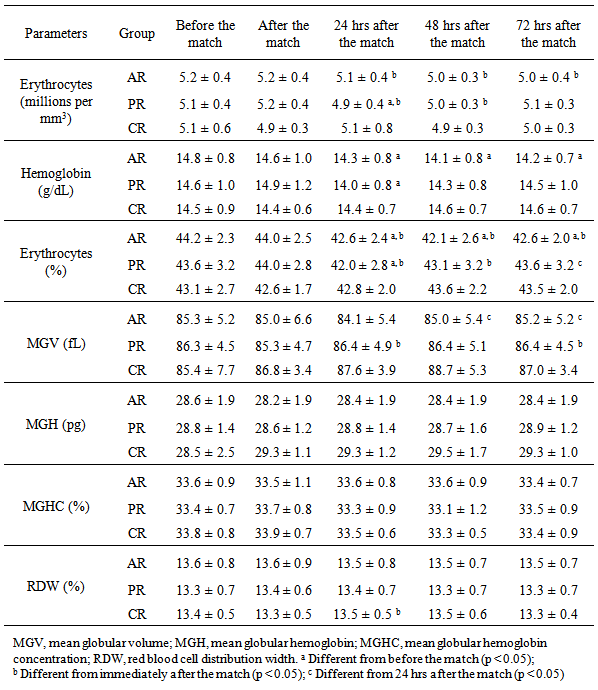
- For blood collection, the participants were asked to sit on a chair. Antecubital fossa skin was cleaned with 95% alcohol, and 5 mL of blood from the arm vein were collected into K3 EDTA-coated tubes (Greiner Bio-One, Austria). The collection tubes were immediately refrigerated and transported to the laboratory for hematologic and immunological analysis. A complete blood count was obtained with an automated hematology analyzer (Sysmex XT-1800i™, Sysmex, Japan). The following parameters were analyzed: Hg, Hct, MGV, MGH, MGHC, red blood cell distribution width (RDW), and erythrocyte, leukocyte, neutrophil, monocyte, and lymphocyte count.
2.4. Statistical Analysis
- Descriptive data are presented as means ± standard deviations. Data normality was checked with Shapiro–Wilk test. Comparisons between groups were performed with repeated measures analysis of variance (ANOVA), and Bonferroni stepwise adjustment was applied for post-hoc comparisons. SPSS™ version 18.0 was used for all analyses. Statistical significance was set at p < 0.05.
3. Results
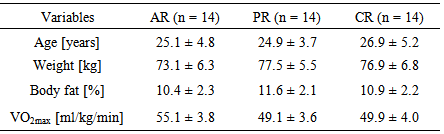
- The participant demographics are presented in Table 1. There were no significant differences between groups in terms of age, weight, percentage of body fat, and VO2max.
|
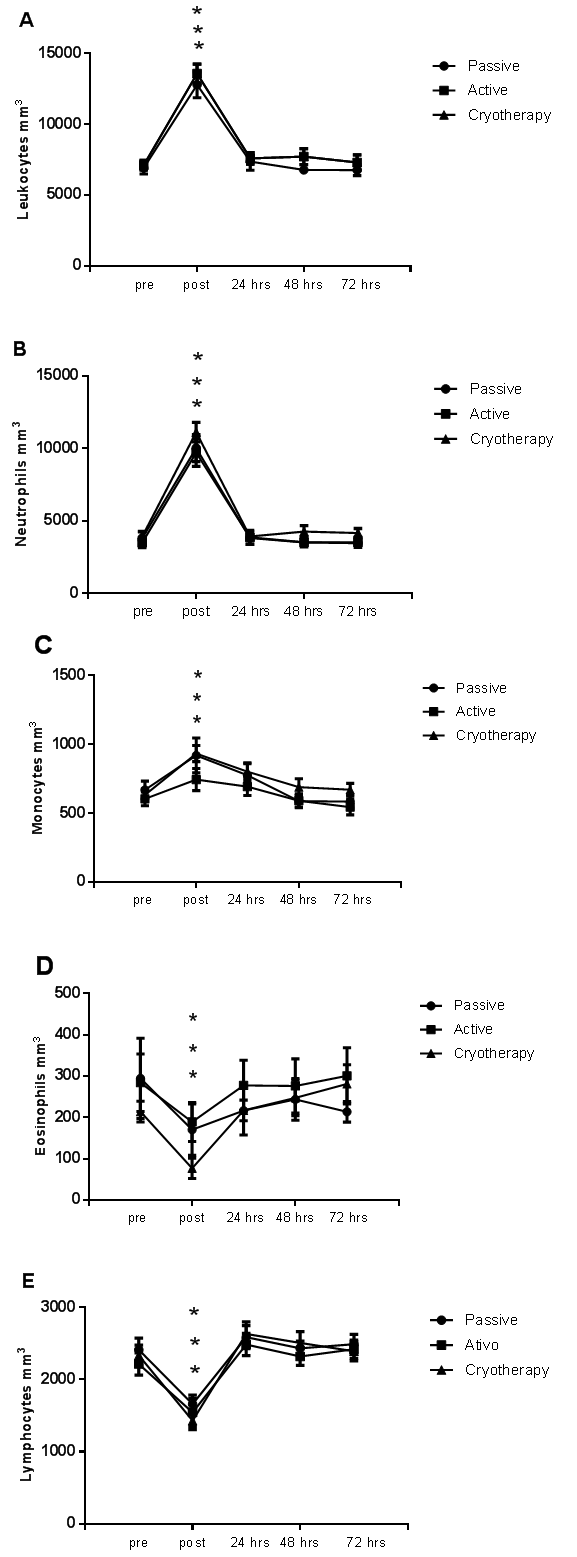 | Figure 1. Cell count of leukocytes (A), neutrophils (B), monocytes (C), eosinophils (D), and lymphocytes (E) obtained before (pre), immediately after (post), and 24 hrs (3), 48 hrs (4), and 72 hrs (5) post-match in soccer players undergoing active recovery, passive recovery, or cryotherapy. No changes in blood cell count were found between groups. * p < 0.05 |
4. Discussion
- Intense physical exercise generally induces hemoconcentration, with consequences for several hemogram parameters. Changes in hematocrit with exercise stress have been reported [29]. However, in the present study, no significant changes in hematocrit were observed immediately after completing a soccer match. One possible explanation for this discrepancy is that in the present study, the soccer players were allowed to drink water ad libitum during and after the match; this might have impeded dehydration and prevented hemoconcentration. We did not measure changes in plasma volume, but it is plausible that hydration ad libitum might have contributed to normal plasma volume and hematocrit. This is supported by work of Knechtle et al. [30], who found increased plasma volume and reduced hematocrit after a 100-km ultra-marathon despite a significant decrease in body weight.Nonetheless, our findings are in agreement with those of previous studies with soccer players [31] and ultra-marathon runners [32]. In both studies, changes in erythrocytes, hemoglobin, and hematocrit were considered clinically non-significant.We observed a slight decrease in erythrocytes, hemoglobin, and hematocrit at 24 hrs post-match in PR and AR. This might have occurred due to hemodilution subsequent to expansion of extracellular fluid volume and increase in plasma volume [33]. In fact, Karakoc et al. [4] observed that after a 90-min training session performed two days after a match, the soccer players presented a decrease in blood viscosity, and subsequent decrease of hemoglobin and hematocrit.The decrease in erythrocyte count and hemoglobin and hematocrit levels at 24 hrs post-match in PR and AR might also be related with mechanical stress: the jumps, falls, tackles, and crashes of a soccer match might overstress both footstrike and intravascular hemolysis [34]. In a study involving 851 athletes, Schumacher et al. [35] found low hematologic indices in endurance runners but not in cyclists, suggesting that the mechanical pattern of running is more traumatic and hemolytic than cycling.Several hematologic parameters (e.g. hematocrit, hemoglobin, and RDW) change throughout the competitive season [33]. Ostojic & Ahmetovic [6] observed higher hematocrit values during the preseason. In a three-year study with 27 soccer players, Malcovati et al. [2] also reported higher hemoglobin and hematocrit values at the start of the competitive season (from June to September) compared with the rest of the season (from October to January), possibly due to higher blood dilution during the latter period. It is important to note, however, that in our study changes in erythrocytes, hemoglobin, and hematocrit were considered to be clinically non-significant.Regarding the immune function, we observed an increase in the leukocyte population after the match. This mainly reflected an increase in neutrophils and monocytes. However, the values returned to baseline levels within 24 hrs after the match; this was irrespective of the recovery protocol. Other studies reported similar results [3, 5, 36]. Match-induced leukocytosis (with neutrophilia and lymphopenia) was also in accordance with the observations of Gravina et al. [7]. Leukocytosis is a normal acute response usually due to an increase in neutrophils. Leukocytosis depends on the duration, intensity, and type of exercise [36-38]. High plasma levels of hormones (e.g. adrenaline, cortisol, growth hormone, and prolactin) can also induce leukocytosis due to immunomodulatory capacity [36].The neutrophilia observed in our athletes followed the temporal pattern of an acute neutrophil response: neutrophil count typically rises above baseline values immediately after exercise to remain high for 120 min [39]. This response is due to neutrophil demargination, namely high cardiac output induced by high catecholamine and cortisol levels [38, 40]. A similar pattern is observed for monocytes [41, 42]. In the present study, we observed a transient monocytosis that might have been related with macrophage activation during the inflammatory response [43].The lymphopenia observed after the match is in accordance with other studies. Nagatomi [44] suggested that lymphopenia is caused by increased glucocorticoid release. Additionally, Shinkai et al. [45] reported that lymphopenia occurs concomitant with a rise in plasma cortisol level. Moreover, during exercise, the skeletal muscle tissue is disrupted. Altogether, these observations support the close link between exercise and immune function.Interestingly, all three protocols induced similar immunological response after the match. However, it is generally accepted that post-match active recovery with low-intensity exercise has beneficial effects [16, 17]. Fairchild et al. [46] reported that active recovery performed at 30–60% VO2max during at least 15 min promotes more rapid return to baseline lactate levels than passive recovery. In addition, Suzuki et al. [47] have shown the importance of low-intensity exercise performed during the recovery period on psychological recovery and relaxation.Despite a lack of effect on hematologic and immunological biomarkers, cryotherapy promoted the normalization of Hb and Hct levels, suggesting its potential benefits in recovery. On the contrary, Hb and Hct levels were decreased in PR and AR at 24 hrs post-match. These findings are supported by Banfim et al. [48], who showed that cryotherapy could be useful to decrease exercise-induced hemolysis.Nevertheless, players subjected to cryotherapy had slightly higher leukocyte cell numbers up to 72 hrs post-match compared to players in PR and AR, which suggests the effect of cold-water immersion in the activation of the immune system [49, 50]. In this line, Stacey et al. [51] observed that, compared to active and passive recovery, cyclists responded well to cryotherapy, with increased immune-system blood markers (neutrophils and lymphocytes) and higher perceived exertion scores, i.e., reduced symptoms of delayed-onset muscle soreness. According to recent studies [23, 24], reduced delayed-onset muscle soreness could be the result of a reduction in nervous conduction velocity and muscle spindle activity, which break the pain–spasm–pain cycle, thus exerting a short-term analgesic effect. However, it is important to know that the beneficial effects of neutrophil and lymphocyte rising during the recovery process are still unknown. In fact, a highly activated immune system is an indication of ongoing skeletal muscle disruption.The currently used hematologic and immunological markers might not be the best choice for monitoring the advantages of recovery training protocols. Another limiting factors might be the 24-hr interval between match and data collection, which could be outside the “window of activity” of the biomarkers tested. Further analyses with a broader range of markers and a larger number of players, and with data collected within the 24 hrs post-match, are warranted.
5. Conclusions
- A single soccer match was not sufficient to induce significant acute changes in hematologic parameters. The changes in erythrocytes, hemoglobin, and hematocrit during the recovery period were significant but clinically non-relevant. On the other hand, immune cell counts (marked leukocytosis with neutrophilia and lymphopenia) were significantly changed post-match. The reverse of the immunological response induced after the match was irrespective of the recovery strategy. However, the hematologic and immunological markers herein used might be limited in detecting differences between recovery strategies. Therefore, future studies using other physiological parameters are needed to further investigate the differences between active, passive, and cryotherapy recovery strategies.
References
| [1] | Andrzejewski, M., Domaszewska, K., Chmura, J., Rychlewski, T., Kubalewska, M., 2008, Influence of speed training loads on the activity of creatine kinase and lactic dehydrogenase and the concentration of oxypurines in blood samples of young football players, Medycyna Sportowa, 24, 149–158. |
| [2] | Malcovati, L., Pascutto, C., Cazzola, M., 2003, Hematologic passport for athletes competing in endurance sports: a feasibility study, Haematologica, 88, 570–581. |
| [3] | Malm, C., Ekblom, O., Ekblom, B., 2004, Immune system alteration in response to two consecutive soccer games, Acta Physiologica Scandinavica, 180, 143–155. |
| [4] | Karakoc, Y., Duzova, H., Polat, A., Emre, M. H., Arabaci, I., 2005, Effects of training period on haemorheological variables in regularly trained footballers, British Journal of Sports Medicine, 39, e4. |
| [5] | Takahashi, I., Umeda, T., Mashiko, T., Chinda, D., Oyama, T., Sugawara, K., Nakaji, S., 2007, Effects of rugby sevens matches on human neutrophil-related non-specific immunity, British Journal of Sports Medicine, 41, 13–18. |
| [6] | Ostojic, S. M., and Ahmetovic, Z., 2009, Indicators of iron status in elite soccer players during the sports season, International Journal of Laboratory Hematology, 31, 447–452. |
| [7] | Gravina, L., Ruiz, F., Lekue, J. A., Irazusta, J., and Gil, S. M., 2011, Metabolic impact of a soccer match on female players, Journal of Sports Science, 29, 1345–1352. |
| [8] | Jastrzębski, Z., and Przybylski, W., 2008, A character of the typical training microcycle in footballers during a competition period, Research Yearbook, 14(2), 78–84. |
| [9] | McLellan, C. P., Lovell, D. I., Gass, G. C., 2010, Creatine kinase and endocrine responses of elite players pre, during, and post rugby league match play, Journal of Strength and Conditioning Research, 24, 2908–2919. |
| [10] | Fallon, K. E., Fallon, S. K., Boston, T., 2001, The acute phase response and exercise: court and field sports, British Journal of Sports Medicine, 35, 170–173. |
| [11] | Nédélec, M., McCall, A., Carling, C., Legall, F., Berthoin, S., Dupont, G., 2012, Recovery in soccer: part I - post-match fatigue and time course of recovery, Sports Medicine, 42(12), 997–1015. |
| [12] | Kraemer, W. J., Spiering, B. A., Volek, J. S., Martin, G. J., Howard, R. L., Ratamess, N. A., Hatfield, D. L., Vingren, J. L., Ho, J. Y., Fragala, M. S., Thomas, G. A., French, D. N., Anderson, J. M., Häkkinen, K., Maresh, C. M., 2009, Recovery from a national collegiate athletic association division I football game: muscle damage and hormonal status. Journal of Strength and Conditioning Research, 23(1), 2–10. |
| [13] | Camargo, M. Z., Siqueira, C. P., Preti, M. C., Nakamura, F. Y., de Lima, F. M., Dias, I. F., Toginho Filho, D. O., Ramos, S. P., 2012, Effects of light emitting diode (LED) therapy and cold water immersion therapy on exercise-induced muscle damage in rats, Lasers in Medical Science, 27(5), 1051–1058. |
| [14] | Bailey, D. M., Erith, S. J., Griffin, P. J., Dowson, A., Brewer, D. S., Gant, N., Williams, C., 2007, Influence of cold-water immersion on indices of muscle damage following prolonged intermittent shuttle running, Journal of Sports Science, 25(11), 1163–1170. |
| [15] | Sellwood, K. L., Brukner, P., Williams, D., Nicol, A., Hinman, R, 2007, Ice-water immersion and delayed-onset muscle soreness: a randomised controlled trial, British Journal of Sports Medicine, 41(6), 392–397. |
| [16] | Cheung, K., Hume, P., Maxwell, L., 2003, Delayed onset muscle soreness: treatment strategies and performance factors, Sports Medicine, 33(2), 145–164. |
| [17] | Cochrane, D. J., 2004, Alternating hot and cold water immersion for athlete recovery: a review, Physical Therapy in Sport, 5(1), 26–32. |
| [18] | Coffey, V., Leveritt, M., Gill, N., 2004, Effect of recovery modality on 4-hour repeated treadmill running performance and changes in physiological variables, Journal of Science and Medicine in Sport, 7(1), 1–10. |
| [19] | Spierer, D. K., Goldsmith, R., Baran, D. A., Hryniewicz, K., Katz, S. D., 2004, Effects of active vs. passive recovery on work performed during serial supramaximal exercise tests, Internaltional Journal of Sports Medicine, 25(2), 109–114. |
| [20] | Gill, N. D., Beaven, C. M., Cook, C., 2006, Effectiveness of post-match recovery strategies in rugby players, British Journal of Sports Medicine, 40(3), 260–263. |
| [21] | Burgess, T. L., Lambert, M. I., 2010, The efficacy of cryotherapy on recovery following exercise-induced muscle damage, International SportsMed Journal, 11(2), 258–277. |
| [22] | Kaczmarek, M., Mucha, D., Jarawka, N., 2013, Cold water immersion as a post-exercise recovery strategy, Medicina Sportiva, 17(1), 35–39. |
| [23] | Bleakley, C., McDonough, S., Gardner, E., Baxter, G. D., Hopkins, J. T., Davison, G. W., 2012, Cold-water immersion (cryotherapy) for preventing and treating muscle soreness after exercise, The Cochrane Database of Systematic Reviews, 2:CD008262. |
| [24] | Nédélec, M., McCall, A., Carling, C., Legall, F., Berthoin, S., Dupont, G., 2013, Recovery in soccer: part ii - recovery strategies, Sports Medicine, 43(1), 9–22. |
| [25] | Leeder, J., Gissane, C., van Someren, K., Gregson, W., Howatson, G., 2012, Cold water immersion and recovery from strenuous exercise: a meta-analysis, British Journal of Sports Medicine, 46(4), 233–240. |
| [26] | Kahanov, L., Eberman, L. E., Wasik, M., Alvey, T., 2012, Exertional rhabdomyolysis in a collegiate American football player after preventive cold-water immersion: a case report. Journal of Athletic Training, 47(2), 228–232. |
| [27] | Howatson, G., Goodall, S., Someren, K. A., 2009, The influence of cold water immersions on adaptation following a single bout of damaging exercise. European Journal of Applied Physiology, 105(4): 615–621. |
| [28] | Leger, L. A., D. Mercier, C. Gadoury, J. Lambert, 1988, The multistage 20 metre shuttle run test for aerobic fitness, Journal of Sports Science, 6, 93–101. |
| [29] | Ring, C., Patterson, S. M., Bacon, S. L., Veldhuijzen van Zanten, J. J., Willemsen, G., Carroll, D., 2008, Reliability of hematocrit during rest and stress in healthy adults, Biological Psychology, 77, 63–68. |
| [30] | Knechtle, B., Knechtle, P., Wirth, A., Alexander Rust, C., Rosemann, T., 2012, A faster running speed is associated with a greater body weight loss in 100-km ultra-marathoners, Journal of Sports Science, 30, 1131–1140. |
| [31] | Gravina, L., Ruiz, F., Lekue, J. A., Irazusta, J., Gil, S. M., 2011, Metabolic impact of a soccer match on female players, Journal of Sports Science, 29, 1345–1352. |
| [32] | Lippi, G., Schena, F., Salvagno, G. L., Aloe, R., Banfi, G., Guidi, G. C., 2012, Foot-strike haemolysis after a 60-km ultramarathon, Blood Transfusion, 10, 377–383. |
| [33] | Banfi, G., Lundby, C., Robach, P., Lippi, G., 2011, Seasonal variations of haematological parameters in athletes, European Journal of Applied Physiology, 111, 9–16. |
| [34] | Robinson, Y., Cristancho, E., Boning, D., 2006, Intravascular hemolysis and mean red blood cell age in athletes, Medicine & Science in Sports & Exercise, 38, 480–483. |
| [35] | Schumacher, Y. O., Schmid, A., Grathwohl, D., Bultermann, D., Berg, A., 2002, Hematological indices and iron status in athletes of various sports and performances, Medicine & Science in Sports & Exercise, 34, 869–875. |
| [36] | Gleeson, M., 2007, Immune function in sport and exercise, Journal of Applied Physiology, 103, 693–699. |
| [37] | Nieman, D. C., Henson, D. A., Austin, M. D., Brown, V. A., 2005, Immune response to a 30-minute walk, Medicine and Science in Sports and Exercise, 37, 57–62. |
| [38] | Martins, F. S. B., and Rodrigues dos Santos, J. A., 2012, Alterações agudas induzidas por uma prova de triathlon longo em diferentes biomarcadores enzimáticos e da função imune [Acute changes in different enzymatic and immunological biomarkers induced by long-course triathlon racing], Revista Brasileira de Fisiologia do Exercício, 11, 7–12. |
| [39] | Freidenreich, D. J., and Volek, J. S., 2012, Immune responses to resistance exercise, Exercise Immunology Review, 18, 8–41. |
| [40] | Gabriel, H., Schwarz, L., Steffens, G., Kindermann, W., 1992, Immunoregulatory hormones, circulating leucocyte and lymphocyte subpopulations before and after endurance exercise of different intensities, International Journal of Sports Medicine, 13, 359–366. |
| [41] | Ramel, A., Wagner, K. H., Elmadfa, I., 2003, Acute impact of submaximal resistance exercise on immunological and hormonal parameters in young men, Journal of Sports Sciences, 21, 1001–1008. |
| [42] | Mayhew, D. L., Thyfault, J. P., Koch, A. J., 2005, Rest-interval length affects leukocyte levels during heavy resistance exercise, Journal of Strength and Conditioning Research, 19, 16–22. |
| [43] | Ortega, E., Forner, M. A., Garcia, J. J., Rodriguez, A. B., Barriga, C., 1999, Enhanced chemotaxis of macrophages by strenuous exercise in trained mice: thyroid hormones as possible mediators, Molecular and Cellular Biochemistry, 201, 41–47. |
| [44] | Nagatomi, R., 2006, The implication of alterations in leukocyte subset counts on immune function, Exercise Immunology Review, 12, 54–71. |
| [45] | Shinkai, S., Watanabe, S., Asai, H., Shek, P. N., 1996, Cortisol response to exercise and post-exercise suppression of blood lymphocyte subset counts, International Journal of Sports Medicine, 17, 597–603. |
| [46] | Fairchild, T. J., Armstrong, A. A., Rao, A., Liu, H., Lawrence, S., Fournier, P. A., 2003, Glycogen synthesis in muscle fibers during active recovery from intense exercise. Medicine & Science in Sports & Exercise, 35(4), 595–602. |
| [47] | Suzuki, M., Umeda, T., Nakaji, S., Shimoyama, T., Mashiko, T., Sugawara, K., 2004, Effect of incorporating low intensity exercise into the recovery period after a rugby match, British Journal of Sports Medicine, 38, 436–440. |
| [48] | Banfi, G., Melegati, G., Barassi, A., d’Eril, G. M., 2009, Beneficial effects of the whole-body cryotherapy on sport haemolysis, Journal of Human Sport & Exercise, 4, 189–193. |
| [49] | Jansky, L., Pospisilova, D., Honzova, S., Ulicny, B., Sramek, P., Zeman, V., Kamínková, J., 1996, Immune system of cold-exposed and cold-adapted humans, European Journal of Applied Physiology and Occupational Physiology, 72, 445–450. |
| [50] | Brenner, I. K., Castellani, J. W., Gabaree, C., Young, A. J., Zamecnik, J., Shephard, R. J., Shek, P. N., 1999, Immune changes in humans during cold exposure: effects of prior heating and exercise, Journal of Applied Physiology, 87, 699–710. |
| [51] | Stacey, D. L., Gibala, M. J., Martin Ginis, K. A., Timmons, B. W., 2010, Effects of recovery method after exercise on performance, immune changes, and psychological outcomes, Journal of Orthopaedic & Sports Physical Therapy, 40, 656–665. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML