-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2014; 4(A): 25-31
doi:10.5923/s.microbiology.201401.04
Mycobacterium tuberculosis and Molecular Epidemiology: An Overview
Asho Ali
Department of Biology, King Abdul Aziz University, Jeddah, KSA
Correspondence to: Asho Ali , Department of Biology, King Abdul Aziz University, Jeddah, KSA.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Tuberculosis is a disease of grave concern which infects one-third of the global population. The high incidence of tuberculosis is further compounded by the increasing emergence of drug resistant strains including multi-drug resistant (MDR). Global incidence MDR-TB is ~4%. Molecular epidemiological studies, based on the assumption that patients infected with clustered strains are epidemiologically linked, have helped understand the transmission dynamics of disease. It has also helped to investigate the basis of variation in Mycobacterium tuberculosis (MTB) strains, differences in transmission, and severity of disease or drug resistance mechanisms from across the globe. This has helped in developing strategies for the treatment and prevention of the disease including MDR.
Keywords: Mycobcaterium tuberculosis, Molecular epidemiology, Drug resistance
Cite this paper: Asho Ali , Mycobacterium tuberculosis and Molecular Epidemiology: An Overview, Journal of Microbiology Research, Vol. 4 No. A, 2014, pp. 25-31. doi: 10.5923/s.microbiology.201401.04.
Article Outline
- “If the importance of a disease for mankind is measured by the number of fatalities it causes, then tuberculosis must be considered much more important than those most feared infectious diseases, plague, cholera and the like. One in seven of all human beings dies from tuberculosis. If one only considers the productive middle-age groups, tuberculosis carries away one-third, and often more.”
 Robert Koch, March 24, 1882
Robert Koch, March 24, 18821. Introduction
- More than 130 years after the discovery of its causative agent, tuberculosis (TB) is still a major killer disease worldwide. It is estimated that today one third of the world population is infected with TB. According to World Health Organization (WHO) estimates in 2012, > 8 million people developed TB and 1.3 died due to the disease [1]. The incidence of TB ranges from less than 10 per 100,000 in North America to 100 to 300 per 100,000 in Asia and Western Russia to over 300 per 100,000 in Southern and Central Africa [1]. With the introduction of antituberculosis therapy (ATT) in1950s and the use of Bacille Calmette Guerin (BCG) vaccine, many experts calculated that TB would be eradicated in few years. However, TB generally and drug resistant TB especially is increasing worldwide since 1990. WHO declared TB a global emergency in 1993. Although, globally the prevalence and mortality rate has fallen by 37% and 45% respectively since 1990, the number of TB cases and deaths are still high. WHO has identified 22 highly endemic countries, which contribute about 80% to the global TB burden. These countries are labeled as being high burden countries (HBCs) of TB. Drug resistant MTB is a major obstacle for the control and successful treatment of tuberculosis (TB). Inappropriate use of first and second line drugs leads to emergence of multidrug-resistant tuberculosis (MDR-TB) and extensively drug resistant tuberculosis (XDR-TB). MDR-TB is caused by isolates which are resistant to at least two first line drugs i.e. rifampicin (RIF) and isoniazid (INH). XDR-TB is caused by MDR-TB isolates which are also resistant to any fluoroquinolone, and to one of the three injectable drugs, capreomycin, kanamycin, and amikacin. WHO estimates that among the patients who were reported to have TB in 2011, there were 310,000 incident cases of MDR-TB and more than 60% of these were from Asia, Africa and Russia [2, 3]. According to WHO estimates 3.6% of new TB cases and 20.2% of previously treated cases in 2012 had MDR-TB globally. WHO further estimates that globally MDR-TB has led to 170 000 deaths in 2012 [1]. Thus drug-resistant TB (DR-TB) is a major threat for global TB control. In addition human immunodeficiency virus (HIV) epidemic has also played a key role in increasing the number of new TB cases [1, 4, 5]. Thus global increase in TB, particularly of drug resistant strains emphasize the need for rapid detection and drug susceptibility testing of MTB in clinical samples. Such rapid detection is necessary for adequate antituberculous therapy and containment of resistant strains. Here, molecular epidemiology has greatly assisted in the analysis of disease transmission and rapid and correct diagnosis of drug resistant MTB strains which in turn may result in decreased transmission of sensitive and drug resistant MTB strains in a population.
2. Molecular Epidemiology of Tuberculosis for MTB Strain Typing
- Molecular epidemiology is an integration of molecular biology with epidemiology. Recent developments in molecular biology have resulted in techniques that allow rapid identification and tracking of specific strains of M. tuberculosis as they spread through the population [6-9]. While, previous methods, such as colony morphology, comparative growth rates, susceptibility to antibiotics, and phage typing were useful but did not provide sufficient information regarding TB epidemiology. There is increasing evidence that the genetic difference of MTB is strongly associated with specific geographical locations [9-16]. Thus molecular epidemiological studies in a high TB incidence country may provide unique insights into dissemination dynamics and virulence of the pathogen. In the early 1990s, different molecular methods were described to discriminate between MTB isolates [17, 18]. Most of these techniques use DNA polymorphism based on repetitive DNA elements of M. tuberculosis as genetic markers. Each of these methods results in strain specific genetic profiles (fingerprints). Identical strain fingerprints are called clusters, which are usually associated with recent transmission. While strains with unique fingerprints represent remote transmission or infection acquired in past. Several molecular epidemiological studies of tuberculosis have been carried out using various polymorphic repeat sequences i.e. insertion sequence (IS), direct repeat (DR) and tandem repeats (Figure 1). IS elements are small DNA segment that can be inserted at multiple sites. These elements show high level of genetic polymorphism and widely used for studying the genetic variability in MTB species. IS6110-RFLP typing method, based on IS6110 copy numbers and positions, has been used worldwide as the gold standard TB epidemiology because of reproducibility of the results [18]. However, there are certain limitations of this technique. Firstly, around 20% of MTB isolates contains few or no copies of IS6110 element and the method is unreliable for typing such strains. Secondly, it needs around 4.5µg of DNA which takes several weeks to culture enough viable organisms. In addition, the method is labor intensive, technically demanding and expensive (van Soolingen et al, 1995).Various PCR based techniques, which target polymorphic loci other than IS6110, have also been used in epidemiological studies of TB. Spacer oligonucleotide typing (spoligotyping) based on the polymorphism in DR locus is a widely used molecular typing method for epidemiological studies worldwide [19]. The 36 bp size DRs are interspersed by unique spacer DNA sequences of 35-41 bp. Spoligotyping identifies the presence or absence of 43 spacer DNA sequences between the variable direct repeats using PCR in a particular MTB strain. Spoligotyping is simple, rapid and highly reproducible. However, Spoligotyping has less discriminatory power as it targets less than 0.1% MTB genomic area as compared to IS6110 based typing which examines the entire genome (Figure 1). Therefore, it cannot totally replace IS6110-RFLP typing because of its lower discriminatory power, except for in strains with low copy numbers of IS6110 (van Soolingen et al, 2001).
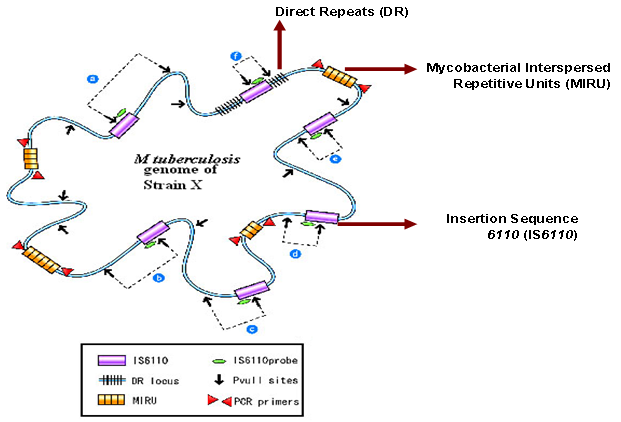 | Figure 1. Hypothetical genome of MTB strain X and polymorphic repetitive sequences such as IS6110, DR and MIRUs for genotyping (adapted from Reference [38]) |
3. Drug Resistance in MTB and Molecular Testing
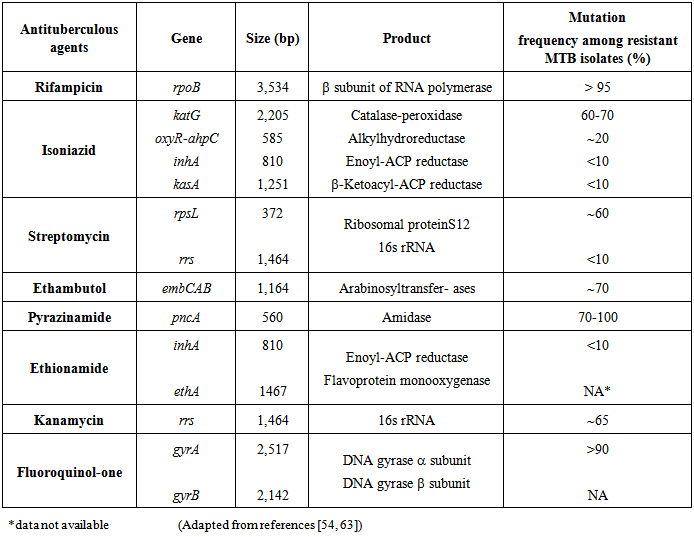
- The resistance of MTB strains to anti-tuberculosis drugs was noted when streptomycin (S) was first used as monotherapy for TB in the 1940s. In the subsequent years with the addition of RMP, pyrazinamide (PYZ) and ethambutol (E), multiple antituberculous therapy was implemented to combat emergence of single drug resistance amongst MTB strains [42, 43]. WHO and International Union against Tuberculosis and Lung Diseases (IUATLD) recommends using two terms: drug resistance among new cases and drug resistance among previously treated cases. Drug resistance among new cases is the presence of drug-resistant strain of M. tuberculosis in a newly diagnosed patient who has never received anti-tuberculosis drugs or has received them for less than 1 month. Drug resistance among previously treated cases is that found in a patient who has previously received at least 1 month therapy with anti-tuberculosis drugs [44].Despite of implementation of multiple antituberculous therapies, a steady increase in the frequency of TB with single and multiple drug resistant MTB strains has been reported throughout the world [45]. In early 1990s outbreaks of MDR-TB received global attention [46]. Nosocomial outbreaks of MDR-TB have been reported in the USA, France and other countries [47, 48]. MDR and XDR-TB can give rise to potentially untreatable form of the disease. MDR and XDR-TB treatment require use of second-line drugs (SLDs) that are less effective, more toxic and more costly than the first line based treatment [49, 50]. In addition mortality is significantly higher among persons infected with MDR and XDR strains than of those infected with sensitive strains. Moreover, patients with MDR and XDR-TB remain infectious for longer time increasing the risk of disease transmission [51, 52].The mechanisms of drug resistance in MTB are chromosomal, caused by accumulation of one or more mutations in independent genes (Table 1). Accumulation of a number of drug resistance mutations result in multiple drug resistance [53]. Such as resistance to RMP is well characterized and more than 95% of the RMP resistant have mutations in an 81bp hot spot region (codon 507-533), or Rif Resistance Determining Region (RRDR) of the 3534bp rpoB gene [54, 55]. RMP interferes with the transcription and elongation of the RNA by binding to the DNA dependent RNA polymerase [55, 56]. In contrast to RMP, INH resistance is controlled by a more complex genetic system, involving several genes such as katG, inhA, kasA, oxyR and ahpC [57-59]. Although the frequency of mutation at these loci varies between different population, studies show that 70-80% of INH resistance is mostly associated with mutation in codon 315 of katG and inhA genes [58, 59]. Similarly S, PYZ, E and Fluoroquinolones resistance have been linked with mutations in rrs, pncA, embB and gyrA genes respectively (Table 1).
|
4. Conclusions
- Molecular methods could enhance understanding of transmission dynamics of tuberculosis disease and could be used to improve current control programs locally as well as globally. Genotyping information of MTB strains together with epidemiologic investigations may provide important information about the spread of MTB strains by identifying factors related to transmission and progression to tuberculosis disease. This in turn could greatly assist in formulating strategies for control of tuberculosis. Moreover, rapid detection of drug resistance using molecular methods in MTB isolates of TB patients in conjunction of routine susceptibility testing could further assist in timely and adequate use of anti-tuberculosis therapy which could play a pivotal role in treatment and containment of sensitive as well as drug resistant tuberculosis. As Ian Sutherland noted:“one man’s cure is many men’s prevention” [71].
ACKNOWLEDGEMENTS
- The author wish to thank Dr Rumina Hasan and Dr Zahra Hasan, Professors at the Aga Khan University, Pakistan, for providing feedback to improve this review as author’s PhD thesis supervisors.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML