-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2014; 4(6A): 18-24
doi:10.5923/s.microbiology.201401.03
Impact of Extracts of Marine Macroalgae on Multidrug-Resistant Bacteria
Ashwag Al-Zahrani 1, Naje Al-Haj 2, Hanan Omer 3, Awatif Al-Judaibi 3
1Department of food Science, Faculty for Girls, King Abdulaziz University, Jeddah, Saudi Arabia
2Department of Medical Microbiology, Faculty of Medicine and Health Sciences, Sana'a University, Sana'a, Yemen
3Department of Biological Science, Science Faculty for Girls, King Abdulaziz University, Jeddah, Saudi Arabia
Correspondence to: Awatif Al-Judaibi , Department of Biological Science, Science Faculty for Girls, King Abdulaziz University, Jeddah, Saudi Arabia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
New antibacterial bioactive compounds are in constant demand because the adaptation of bacteria to antibiotics makes them ineffective for clinical treatments. We investigated the antimicrobial effects of methanol extracts of Ulva sp., Laurencia sp., Padina sp. and Gracilaria changii against the following multidrug-resistant bacteria isolated from patients in Saudi Arabia and Malaysia: Staphylococcus aureus (STR 9), S. aureus (STR 5), S. aureus (N8), S. aureus -20, S. saprophyticus, S. epidermidis, Streptococcus agalactiae (group B), S. Pyogenes, Enterococcus faecalis, Psedomonas aeruginosa, Stenotrophomonas maltophilia, Salmonella typhimurium, Shigella sonnei, Proteus vulgaris, P. mirabilis, Klebsiella pneumonia, Enterobacter aerogenes and E. coli. We found high growth inhibition of S. aureus, S. saprophyticus, S. epidermidis, E. faecalis and P. vulgaris after treatment with Ulva sp., and of S. aureus (STR 5) and S. aureus (N8) after treatment with G. changii; whereas Laurencia sp. and Padina sp. were most effective against S. saprophyticus. We determined minimum inhibitory concentrations of 0.5, 2, 2 and 4 µg mL-1, and minimum bactericidal concentrations of 2, 4, 16 and  32 µg mL-1 for Ulva sp., Laurencia sp., G. changii and Padina sp. respectively. We recommended obtaining new sources of antimicrobial agents from marine algae.
32 µg mL-1 for Ulva sp., Laurencia sp., G. changii and Padina sp. respectively. We recommended obtaining new sources of antimicrobial agents from marine algae.
Keywords: Resistant bacteria, Antimicrobial agents, Seaweed, Growth inhibitory, Antibiotic susceptibility
Cite this paper: Ashwag Al-Zahrani , Naje Al-Haj , Hanan Omer , Awatif Al-Judaibi , Impact of Extracts of Marine Macroalgae on Multidrug-Resistant Bacteria, Journal of Microbiology Research, Vol. 4 No. 6A, 2014, pp. 18-24. doi: 10.5923/s.microbiology.201401.03.
Article Outline
1. Introduction
- Recently, much attention has been paid to seaweeds as a source of bioactive compounds [1-5], some of which have especially high activity [6]. Studies of the antibacterial activities of algae against pathogenic bacteria and fungi in humans have shown evidence of antimicrobial activity in Ulva lactuca, Padina gymnospora, P. Sanctae-cruces, Sargassum wightii, Caulerpha sertularioides and Gracilaria edulis against Staphylococcus aureus, S. epidermidis, Bacillus subtilis, B. cereus, B. anhtracis, Lactobacillus acidophilus, Pseudomonas aeruginosa, E. coli., Proteus mirabilis, Salmonella typhi, Shigella flexneri, Vibrio alginoyticus, Candida albicans and Aspergillus niger [7-12]. An extract of the green algae Caulepra prolifera was reported to demonstrate significant activity against strains of marine bacteria [13].Antibacterial activity has also been screened with equal (v/v) chloroform:methanol extracts of Portieria hornemanii, which inhibited the growth of Xanthomonas axonopodis pv. citri and X. campestris pv. malvalearum, and with gas chromatography–mass spectrometry analysis, which found two potent bioactive compounds in the chloroform extract: 1,2-benzene dicarboxylic acid, diisoctyl ester and 1,2-benzene dicarboxylic acid, bis (2-methyl propyl) ester [14]. Furthermore, the antimicrobial activity of U. lactuca was reported to be caused by acrylic acid, commonly found in algae [15]. Marine organisms synthesize complex organic compounds with antibacterial activity from a diverse set of biological precursors. Interesting biological activities against microorganisms have been demonstrated from a variety of bioactive compounds in algae including sterols, terpenoids and brominated phenolics [16-18]. G. edulis extracted with methanol, acetone, petroleum ether, hexane and ethanol solvents has been found to be effective against K. pneumoniae, Enterobacter aerogens, S. typhi, P. aeruginosa, E. coli, V. cholerae, S. pyogenes B. subtilis, S. aureus, S. epidermis and B. cereus. Methanol extracts were more effective than extracts using other solvents in their inhibitory effects against both gram negative and positive bacteria [19]. In a study of algae collected from the Pacific coast of Chile, gas chromatography–mass spectrometry analysis of dichloromethane extracts of Ceramium rubrum found fatty acids, fatty acid esters, a hydrocarbon and phytol. The results of antimicrobial activity showed that the activity of the crude extract was stronger than its components [20].The synthesis of active compounds that may be used as antimicrobial agents varies among seasons and locations. For example, the algae extract Ulva pertusa Kjellman, which is used against Gardnerella vaginalis, was active when collected during early winter to middle spring, but showed negative activity when collected during summer and autumn [21].Algae in the Red Sea are adapted to living in an environment where the temperature is often above 35˚C, and the cellular composition of algae is defined by available nutrients [22]. The marine environments of the west coast of Saudi Arabia by the Red Sea have not been thoroughly explored; few studies have investigated the potential sources of bioactive compounds in Red Sea algae. In a recent study, Turbinaria triquetra and Halimeda opuntia were collected from the Red Sea and extracted with methanol, ethanol, petroleum ether and dimethyl formamide, and then investigated against E. coli, S. typhi, S. dysenteriae, K. pneumoniae and E. aerogenes. T. triquetra extracted with methanol strongly affected the growth of the tested bacteria; this strong inhibition of growth was reflected on the bacterial potassium leakage, the cell wall service detected by electron scanning microscopy and energy dispersive x-ray analysis that let the bioactive compounds in T. triquetra a potential source of natural antibacterial compounds [23].Our study evaluated the antimicrobial activity of marine algae collected from two different locations, the Red Sea and the Straits of Malacca. We determined the antibacterial activity of Ulva sp., Laurencia sp., Padina sp. and Gracilaria changii extracted with methanol on isolated bacteria collected from patients at several hospitals in Saudi Arabia and Malaysia.
2. Materials and Methods
2.1. Study Material
- The following bacteria were isolated and identified at the King Abdulaziz University Hospital in Saudi Arabia: Staphylococcus aureus, S. saprophyticus, S. epidermidis, Streptococcus agalactiae (group B), S. Pyogenes, Enterococcus faecalis, Psedomonas aeruginosa, Stenotrophomonas maltophilia, Salmonella typhimurium, Shigella sonnei, Proteus vulgaris, P. mirabilis, Klebsiella pneumoniae, Enterobacter aerogenes and E. coli. Additional strains S. aureus (STR 9), S. aureus (STR 5), S. aureus (N8), S. aureus -20, S. pyogenes, E. coli, K. pneumoniae and P. aeruginosa were isolated and identified in Malaysian hospitals (Tengku Ampuan Afzan Hospital, Kuantan Hospital, Seremban Hospital, Miri Sarawak Hospital, Sains Malaysia Kota Bharu University Hospital, Malaya University Medical Centre and Gribbles Pathology Laboratory in Petaling Jaya). We prepared assay plates by inoculating 100 μL-1 of each sample (1×105 colony-forming units) onto Mueller-Hinton agar (OXOID CM 337).The marine algae Ulva sp. (Chlorophyta), Padina sp. (Phyeophyta) and Laurencia sp. (Rhodophyta) were collected from the Red Sea near Jeddah and identified in the Biological Sciences Department in the King Abdulaziz University. Gracilaria changii (Rhodophyta) were collected from Pantai Morib along the Straits of Malacca in Selangor by Dr. Phang Siew Moi from Malaya University, Malaysia. Dr. Habsah Mohamad from Kolej University in Dan Sains Teknologi Marin, Malaysia, provided the methanol extract of G. changii in the form of a paste. The algae were washed with distilled water several times, spread on plates and dried at 40°C. After drying, we ground and solubilized the algae in methanol at 100 g 100 mL-1. The mixtures were kept on a 120 rpm shaker at 30°C for 24 h and then filtered using Whatman No. 1 filter paper. We dried the filtered solvent under reduced pressure at 40°C, and used the resultant deposits as crude extracts [24].
2.2. Laboratory Analyses
- We assessed the antimicrobial susceptibility of the isolated bacteria using the modified Kirby-Bauer disc diffusion method according to the Clinical and Laboratory Standards Institute [25]. We tested the following concentrations of antibiotics: 10 and 50 µg gentamycin, 30 and 50 µg vancomycin, 10 and 25 µg penicillin, 15 μg erythromycin, 50 µg tobramycin and 5 μg methicillin. We tested the resistance of S. auerus to methicillin with 1 µg oxacillin and 2% NaCl. We incubated the bacteria at 37°C for 24 h and measured the inhibition zones of each disc. We performed all tests in triplicate.We determined the antimicrobial activity of each crude algae extract against the gram-positive methicillin-resistant S. aureus in vitro. We measured the activity of each extract by the disc diffusion and broth dilution methods following Clinical and Laboratory Standards Institute protocols [26]. For the disc diffusion method, each extract was dissolved in dimethylsulfoxide at 3 μg mL-1 and filtered through a 0.22 μm pore filter (Millipore, Billerica, MA). We placed 100 μL of each filtered solution on 1 mm diameter paper discs, which were then set on a pre-inoculated agar surface. We prepared negative controls with each solvent. We incubated the plates at 37°C for 24 h and measured the inhibition zones of each disc. We performed all tests in triplicate.We tested the extracts that inhibited the growth of bacteria to determine the minimum inhibitory concentration (MIC) using the broth microdilution method [27]. Bacteria were cultured overnight on Mueller-Hinton agar and then suspended in 1 mL-1 of Mueller-Hinton broth (OXOID CM 405) to give a final concentration of 5 × 105 colony-forming units mL-1. We serially diluted each extract with Mueller-Hinton broth in a 96-well microplate. The plates were inoculated with the bacteria and incubated at 37°C for 16-20 h. After incubation, we evaluated the plates for the visible presence or absence of microbial growth. The MIC was determined as the lowest concentration of an extract for which there was no visible growth compared to the control [28].We determined the minimum bactericidal concentration (MBC) by inoculating 0.1 mL of broth from the negative growth wells in the MIC assay onto sterile nutrient agar using streak plates. We incubated the plates at 37°C for 24 h. We considered the MBC to be the concentration that showed no growth of the tested organisms; the negative control was a plate that contained only medium [29-31].
3. Results
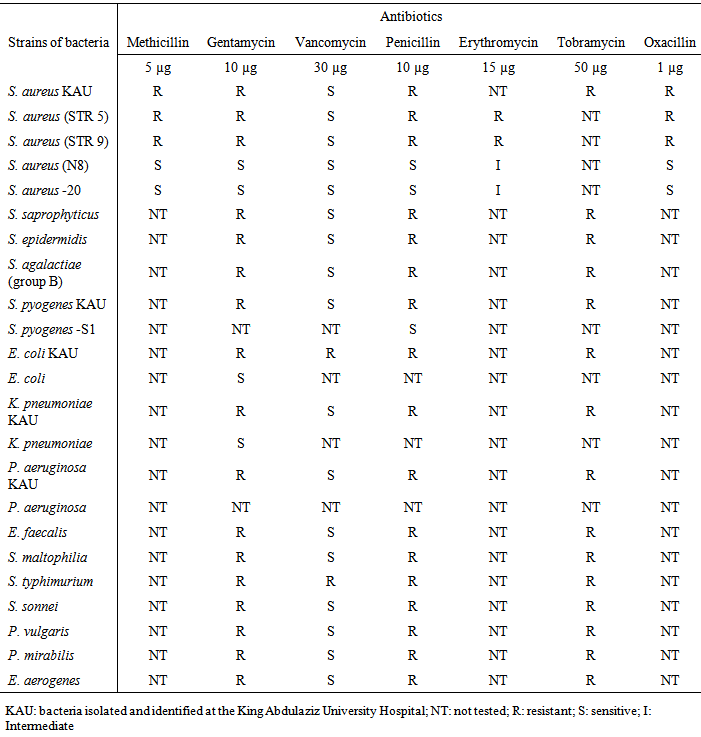
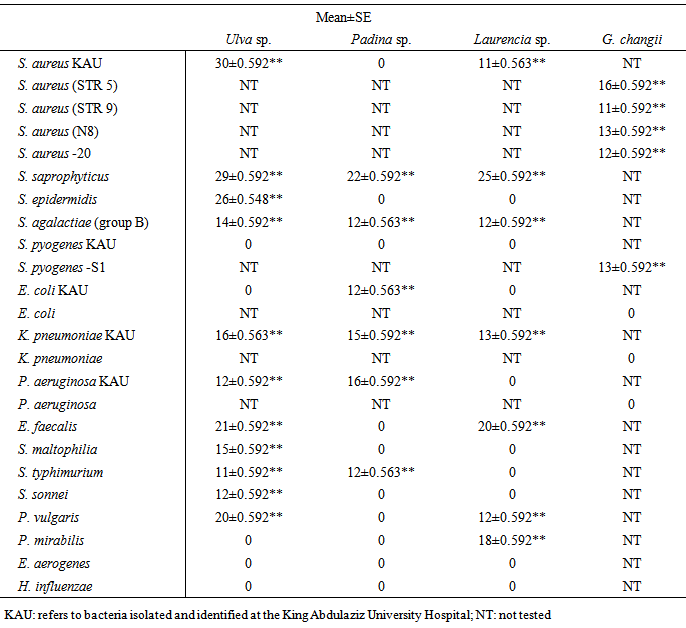
- Our assessment of antimicrobial susceptibility found that the isolated bacteria were resistant to the most of the tested antibiotics except for vancomycin, which was sensitive to most tested bacteria except for E. coli and S. typhimurium isolated from the King Abdulaziz University Hospital (Table 1). Isolated methicillin-resistant S. aureus showed resistance to oxacillin except for the strains S. aureus (N8) and S. aureus -20.
|
|
|
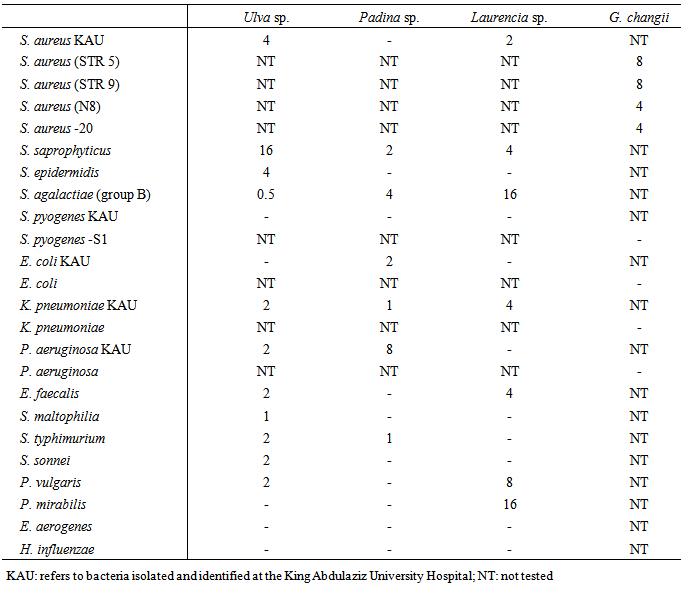
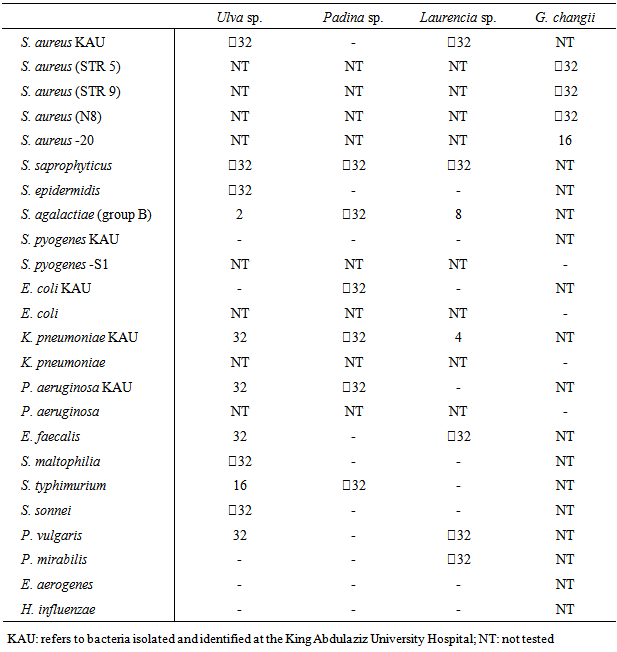
- Our determination of MBCs found values of
 32 µg mL-1 for most tested bacteria (Table 4). The most sensitive bacteria were S. agalactiae (group B) and S. typhimurium after treatment with Ulva sp., K. pneumoniae and S. agalactiae (group B) after treatment with Laurencia sp., and S. aureus -20 after treatment with G. changii.
32 µg mL-1 for most tested bacteria (Table 4). The most sensitive bacteria were S. agalactiae (group B) and S. typhimurium after treatment with Ulva sp., K. pneumoniae and S. agalactiae (group B) after treatment with Laurencia sp., and S. aureus -20 after treatment with G. changii.
|
4. Discussion
- Adaptation of bacteria to antibiotics makes them ineffective, resulting in a constant demand for new antibacterial bioactive compounds. Two different marine environments had potential sources of antibacterial active compounds that can inhibit the growth of multidrug-resistant isolated bacteria. We used isolated bacteria from patients as an indicator of the effectiveness of antibiotics. In our study, most of the isolated bacteria were resistant to the tested antibiotics. This general resistance may be due to a mutation acquired from the uses of these antibiotics. Previous studies of the susceptibility of bacteria to antibiotics have investigated the effects of the antibiotics ciprofloxacin, augmentin, perfloxacin, gentamycin and streptomycin against S. aurous, Salmonella sp. and Klebsiella sp.; imepenem, ticarcillin/clavulanic acid, piperacillin / tazobactum, polymyxin B, piperacillin, amikacin, ceftazidime, tobramycin, ofloxacin and lomefloxacillin against P. aeruginosa; and amoxicillin / clavulanate, cotrimoxazole, ciprofloxacin, cefuroxime, fosfomycin and nitrofurantoin against E. coli, K. pneumoniae, P. mirabilis, S. aureus and E. faecalis. Most of the bacteria were resistant to the antibiotics except for ciprofloxacin, ticarcillin/clavulanic and fosfomycin, which had high activity against gram negative bacteria [20, 32-33]. Algae metabolism is affected by the extreme marine environmental conditions found in our study sites including high pressure, temperature and osmolarity, and the presence of exceptional organic compounds such as isonitrile, dichloramine, isocyanate and halogenated compounds [34].Bacterial growth is inhibited in the presence of antibacterial agents due to their combination with bioactive compounds, which could be secondary metabolism components such as alkaloids, peptides, terpenes, pigments and sterols [35]. Studies have discovered bioactive compounds in the sea algae C. rubrum, P. hornemanii, Ulva sp., Laminaria sp, Fucus sp., Ascophyllum nodosum, Chondrus crispus, Porphyra sp., Sargassum sp., Gracilaria sp., Palmaria palmate and Undaria pinnatifida; the antimicrobial active compounds they detected included alginic acid, fucoidan, laminarin, mannitol, phorphyran, floridean starch and pentoses [36-37]. Studies of fimbrolide extracted from Delisea pulchra against P. aeruginosa and of kahalalide A extracted from Bryopsis sp. against Mycobacterium tuberculosis found high activity of these two active compounds against bacteria [38-39]. High activity of methanol extracts against bacteria, as reflected by the MICs and MBCs that we found, agrees with results of MICs and MBCs after treatment with several organic solvents in other studies [11-12, 23, 40-41].
5. Conclusions
- We found bioactivity in methanol extracts of Ulva sp., Laurencia sp., Padina sp. and Gracilaria changii against isolated multidrug resistant bacteria from patients at hospitals in Saudi Arabia and Malaysia. Our investigation is an example of the potential for obtaining new sources of antimicrobial agents.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML