-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Microbiology Research
p-ISSN: 2166-5885 e-ISSN: 2166-5931
2014; 4(6A): 1-9
doi:10.5923/s.microbiology.201401.01
Antimicrobial Activities and Phytochemical Analysis of the Essential Oil of Ocimum basilicum, Collected from Jeddah Region, Saudi Arabia
Abeer Hamdan Abdullah Ba-Hamdan 1, Magda Mohammad Aly 1, Sameera Omer Bafeel 2
1Biological science-Microbiology section, King Abdulaziz University, Jeddah, Saudi Arabia
2Biological science-Botany section, King Abdulaziz University, Jeddah, Saudi Arabia
Correspondence to: Abeer Hamdan Abdullah Ba-Hamdan , Biological science-Microbiology section, King Abdulaziz University, Jeddah, Saudi Arabia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Multiple drug resistance bacteria have been developed due to the indiscriminate use of commercial antimicrobial drugs commonly used in the treatment of infectious diseases. Thus, it should be searching for new antimicrobial agents from plants and detect its ability to treat diseases caused by resistant micro-organisms. Ocimum basilicum L. (Basil) is one of the most common plants used traditionally all over the world and in Saudi Arabia to treat many diseases. O. basilicum var. Genovese was collected from three different gardens in Jeddah city and the essential oils were extracted with either methanol or ethanol using Soxhlet. The results showed that the methanolic extract of O. basilicum was more active against pathogenic bacteria and fungi compared with the ethanol extract. Antibacterial activity was stronger against Gram positive than Gram negative bacteria. The highest activities were against Bacillus spp., Micrococcus spp. and Staphylococcus aureus. Moderate antifungal activity was recorded against fungi, Fusarium spp. was the most sensitive fungus. No activity was shown against all the tested pathogenic yeasts used in the study. The minimal inhibitory concentration (MIC) for bacteria and fungi were 25-75 µl/ml and 70-150 µl/ml respectively. The extracted oil proved to be effective on the wall composition of the most susceptible bacteria.
Keywords: Antimicrobial activity, Ocimum, Essential oils, GC-MS, MIC
Cite this paper: Abeer Hamdan Abdullah Ba-Hamdan , Magda Mohammad Aly , Sameera Omer Bafeel , Antimicrobial Activities and Phytochemical Analysis of the Essential Oil of Ocimum basilicum, Collected from Jeddah Region, Saudi Arabia, Journal of Microbiology Research, Vol. 4 No. 6A, 2014, pp. 1-9. doi: 10.5923/s.microbiology.201401.01.
Article Outline
1. Introduction
- Recently, multiple drug resistance in both human and plant pathogenic microorganisms have been developed due to the indiscriminate use of commercial antimicrobial drugs commonly used in the treatment of infectious diseases [1, 2]. In addition to this trouble, antibiotics are sometimes associated with negative effects on the host, which includes hypersensitivity, immune-suppression, and allergic reactions [3]. Therefore, there is a need to develop alternative antimicrobial drugs for the treatment of infectious diseases from various sources, including medicinal plants [4]. Many authors reported that, medicinal plants are a source of great economic value all over the world [5-10]. It is estimated that there are between 200,000 and 700,000 species of tropical flowering plants that have medicinal properties [11]. Their actions include antibacterial, antifungal, antiviral, anthelmintic, antiallergic, anticarcinogenic, analysis and larvicidal agents [12]. The beneficial medicinal effects of plant materials may typically result from the combinations of secondary products present in the plant. In plants, these compounds are mostly secondary metabolites such as alkaloids, essential oils, tannins, phenols, flavonoids, steroids, resins fatty acid gums, which are capable of producing definite physiological action on the microorganisms [13] and medically resistant strains of bacteria have been found to be widely inhibited by these compounds [14].Many herb spices, especially those belonging to the Lamiaceae family, such as Ocimum showed strong antioxidant and antimicrobial activities [15]. Various species of Ocimum have been reported for their numerous medical uses [16]. Ocimum plant is widely spread in the world, which includes annual and perennial herbal plants, as well as shrubs, from tropical and subtropical zones of Asia, Africa and Central, and South America. The main center of diversity appears to be in Africa, further about 200 species of herbs and shrubs for this genus [17, 18].The most important species of Ocimum genus are O. sanctum L. and O. basilicum L.; this latter species, usually named common basil or sweet basil and it is characterized by a considerable morphological and biochemical variability [19]. It will grow to a size of 1-2 feet in height [20]. Basil will prolifically produce large green leaves, measuring around 2 inches in length, throughout the summer [21]. Basil flowers are white, days to flowering are 100 days and are commonly removed to increase yield of leaves [18, 22]. It is very important for their therapeutic potential values. Leaves and flowering parts of O. basilicum are traditionally used as antispasmodic, aromatic, carminative, digestive, galactogogue, stomachic and tonic agents [23-25]. The aims of this work were studied activity essential oils which extracted from aerial parts of O. basilicum var. Genovese against some pathogenic microorganisms as an antimicrobial.Minimum inhibitory concentration (MIC) and the chemical composition of the basil oil in addition to its effect on the bacteria cell walls were also studied.
2. Materials and Methods
2.1. Tested Microorganisms
- Tested microorganisms include the fungi Penicillium glabrum, Aspergillus niger, Aspergillus flavus, Fusarium spp. and Cladosporium spp., the yeast Candida albicans and Saccharomyces spp. and the bacteria Methicillin-resistant Staphylococcus aureus (MRSA), Bacillus spp. and Micrococcus spp., Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus epiderimdis, Klebsiella pneumonia, Streptococcus pneumonia and Acinetobacte spp. microorganisms were obtained from King Abdulaziz hospital, faculty of science, King Abdul Aziz University, and King Fahd Hospital, Jeddah, Saudi Arabia. The tested bacteria strains were maintained on the slopes of nutrient agar at 4ºC [26]. All the organisms were regenerated every six months.
2.2. Tested Plant Extraction
- Arial parts including fresh leaves, flowers and wooden parts of Ocimum basilicum were collected from three different botanical gardens at north, central and south of Jeddah in Mars 2009, at 8 AM. The collected plant materials were put in clean plastic bags and transferred directly to the lab. The collected materials were cleaned and the infected or dead parts were excluded. The plant parts used were washed thoroughly using tap water and left to dry at room temperature for seven days. Then, the dried plant was pulverized using mechanical grander. The powder was sieved with a 2 mm diameter mesh.The plant was identified as Ocimum basilicum at Faculty of Science, Biology Department, King Abdul Aziz University. The complete identification was carried out at Botany Department, Faculty of Science, Tanta University Egypt, as O. basilicum var. Genovese.By few modifications of [27] method: the dried and powdered plant materials (300 g) were extracted with 1 liter of either methanol or ethanol using Soxhlet (Electromantle ME) for 12 h at 90ºC to obtain the essential oil of the plant. Both methanolic and enthanolic extracts were concentrated by evaporating to dryness using rotary evaporator (Heidolph) at 40ºC. The essential oils obtained were collected and maintained according to [28] at 4ºC until used in hermetically sealed dark glass bottles with rubber lids, covered with aluminium foil to protect the contents from light and kept under refrigeration at 4ºC until used without any prior purification.
2.3. Antimicrobial Activities of Ocimum basilicum Extract
- This test was carried out according to the method of [9]. 15 ml of sterilized nutrient agar (NA) for bacteria, potato dextrose agar (PDA) for fungi and Sabouraud agar for yeasts were poured into each Petri plate (90 mm diameter) and allowed to solidify. The plates were incubated with freshly prepared inoculums which were swabbed over the entire surface of the medium, rotating the plate 60 degrees after each application by using a sterile cotton swab, to ensure the spread of the tested microbes on the surface of the plate completely. Inoculums were 108 CFU/ml for bacteria, 104 spore/ml for fungi and 106 CFU/ml of yeast compared with 0.5 McFarland densities [29, 30]. One wheel of 6mm diameter was bored with the medium of each plate with the help of sterile cork-borer. 50 μl of essential oil was filled each well with the help of micropipette. Ampicillin (5µg/ml) was used as positive control against bacteria and amphotericin B (5µg/ml) was used as positive control against fungi and yeasts and sterile water as a negative control. Plates were left for 45 min at room temperature to allow proper diffusion of the extract to the medium. All plates were incubated at 37ºC for 24 h, 28ºC for 48 h and 37ºC for 48 h for bacteria, fungi and yeasts respectively, and the inhibition zones were measured (mm). Inhibition of microbial growth was measured as the diameter of inhibition zone (mm) at 3-equidistant points taken from the center of the inhibition zone and the average value was calculated. All experiments were carried out in triplicate.
2.4. Determination of the Minimum Inhibitory Concentration (MIC)
- This method was done as recommended by [31]. About 100 µl from each concentration of the plant extract are added to sterilize microtitre plate containing 100 µl of nutrient broth medium for bacteria and Sabouraud broth medium for fungi. Freshly prepared standard number of cells 106 CFU/ml for bacteria and 104spore/ml for fungi were added plus some drops of phenol red. Growth or glucose metabolism was measured by a change of the color of phenol red indicator from red to yellow. Minimum inhibitory concentrations (MICs) are defined as no change in color of phenol red. For every experiment, sterile distilled water was used as negative control and standard antibiotics were included as a positive control. All experiments were carried out in triplicate.
2.5. Effect of O. basilicum Oil on Bacterial Cell Wall (mode of action)
- The effect of the extracted oil was tested on Bacillus spp. The most sensitive bacteria in the study, cell wall was carried out on a morphological character using scanning electron microscope (SEM) and on the cell wall composition.
2.5.1. Isolation of Bacterial Cell Wall
- The cell wall of Bacillus spp. was obtained using the method, which described by [32]. Bacillus spp. was cultivated in 250 ml Erlenmeyer flask containing 45 ml of nutrient broth, 2 ml bacterial suspension (108 CFU/ml) and 5 ml of the methanolic extract of O. basilicum or 5 ml sterile distilled water as negative control. Then, all flasks were incubated at 37ºC for 24 h. After then, the bacterial cells were collected by centrifugation at 3000 rpm for 15 min to precipitate the cells. Cells were washed two times by sterile water and preservation at low temperatures. The bacterial cell walls were broken using cell Homogenizer (Ultra-Turraxt 25) at low temperature for 5 min three times. After centrifugation at 3000 rpm for 30 min, the cell walls were collected and preserved at -20°C until used.
2.5.1.1. Quantitative Determination of Sugars, Protein and Phosphorous in Cell Wall
- The determination of sugar was performed according to [33]. In the acidic medium, the concentrated sulfuric acid transformed the hexose or pentose sugars to furfural which can be condensed with the phenol to form a brown color complex. About 200 μl of the digested cell walls were mixed with 10 μl of 80% phenol in water and 1.5 ml conc. H2SO4. The tubes were left for 5 min in a boiling water bath and then left at room temperature for 30 min. The absorbance was measured at A485 nm by using spectrophotometer (Perkin Elmer, USA) against a blank (200 μl of the sterile distilled water was mixed with 10 μl of 80% phenol in water and 1.5 ml conc. H2SO4.). The quantity of the sugar can be determined from a standard curve of glucose.The protein content of the cell wall was determined as described by [34]. The assay reagent was made by dissolving 100 mg of Coomassie Blue G250 in 50 ml of 95% ethanol. The solution was mixed with 100 ml of 85% phosphoric acid and made up to 1 L with distilled water. 1ml of the digested cell wall was mixed with 5 ml of assay reagent, mix well and the absorbance was measured at A595 nm. Blank was composed of 5 ml assay reagent plus 1ml sterile distilled water. The quantity of protein was determined from a standard curve of albumin.Phosphor content was determinate spectrophotometerically. One ml of acid digested cell wall was neutralized by 8 N NaOH and one ml of ammonium molybdate sulfuric acid (25g NH4MO2 in 200 ml worm H2O was filtrated above 400 ml of H2O + 280 ml of conc. H2SO4 and completed to one liter) followed by one ml of SnCl2.2H2O (0.5 gm SnCl2 in 250 ml 2% HCl) were added. The contents were diluted to a certain volume, and the density of the color was determined using spectrophotometer at A700 [35]. The quantity was determined from a standard of KH2PO4.
2.5.1.2. Imaging the Cells Using Scanning Electron Microscope (SEM)
- Bacillus spp. was grown in 5ml nutrient broth with 2 ml methanolic oil as a treated sample and 2ml sterile distilled water as negative control. Then, all samples incubated at 37ºC. After centrifugation at 3000 rpm for 30 min the cell walls were collected and prepared for using scanning electron microscopy (Quanta FEG 450) in unit of the electron microscope, King Abdulaziz University, Jeddah, Saudi Arabia.
2.6. Chemical analysis by Gas Chromatography–mass Spectrometry (GC/MS)
- The essential oils of O. basilicum were rich with large number of compounds which considered as secondary metabolites. The chemical analysis was determined using gas chromatography -mass spectrometry (GC/MS) in faculty of Pharmacy, King Abdul Aziz University, Jeddah, Saudi Arabia.
2.7. Statistical Analysis
- Statistical analyses were performed using the Statistical Package for Social Science (SPSS for windows, version 16) (SPSS Inc., Chicago, IL, U.S.A). The varying degree of the result is expressed as mean ± standard deviation (Mean ± SD). The significance of the difference between samples was determined using t-test. The difference was regarded significant when P < 0.05 and nonsignificant when P > 0.05, where P is a level of significance.
3. Results
3.1. Antimicrobial Activities of the Essential Oils
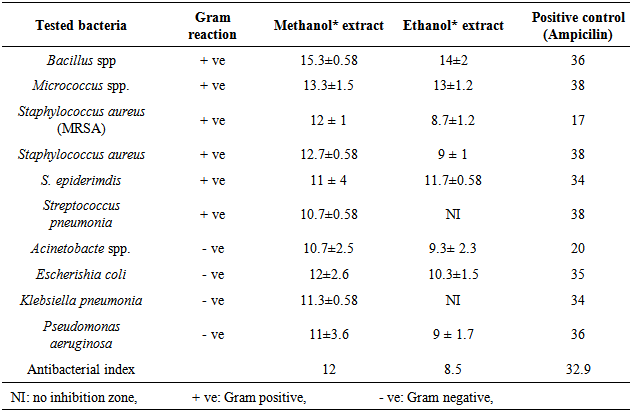
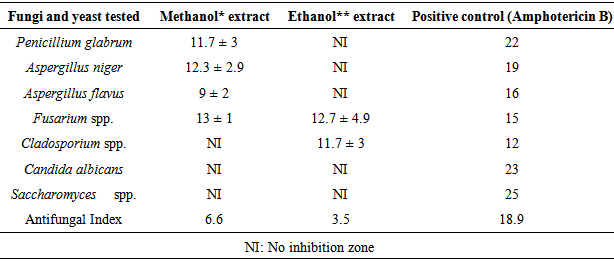
- The results of the inhibit growth of the essential oil on the tested bacteria are shown in Table 1 and Figure 1, the high inhibition was with the methanol extract and the most sensitive bacteria is Bacillus spp. with inhibition of 15.3 mm, then, Micrococcus spp., Staphylococcus aureus (MRSA), S. aureus and E.coli with inhibitions of 12, 12.7 and 12 respectively. In Table 2 and Figure 2 the results showed the inhibition of fungal growth after the treated with methanol or ethanol extracts, Fusarium spp. was the sensitive tested fungi with inhibition of 13 mm, C. albicans and Saccharomyces spp. were more resistant with no inhibition of growth appeared after the treated with both extracted.
|
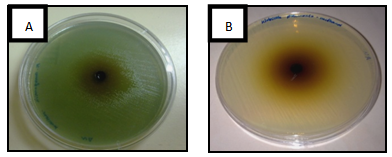 | Figure 1. Antibacterial activity of Ocimum basilicum essential oil on (A) Pseudomonas aeruginosa and (B) Klebsiella pneumonai |
|
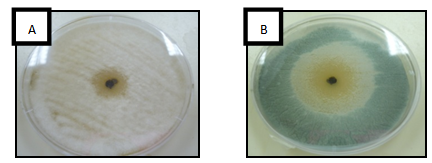 | Figure 2. Antifungal activity of Ocimum basilicum essential oil on (A) Aspergillus flavus and (B) Cladosporium spp |
3.2. Determination of the Minimum Inhibitory Concentration (MIC)
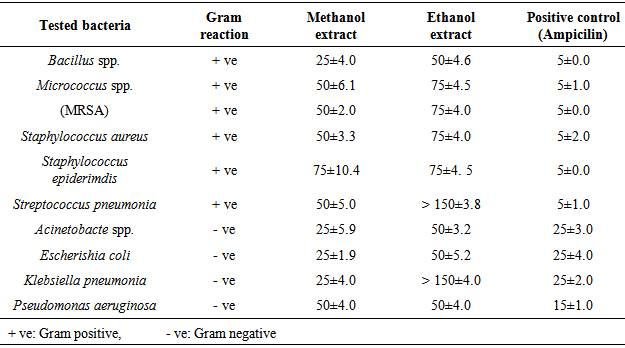
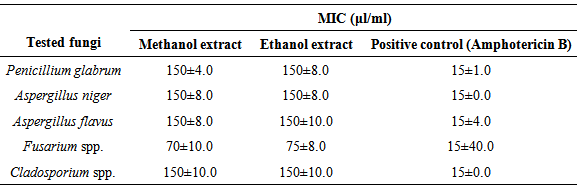
- The results of O. basilicum MICs on tested microorganisms were showed in Tables 3 and 4, Bacillus spp., Acinetobacte spp, E.coli and K. pneumonia have the minimum inhibitory concentration with a value of 25 μl/ml O. basilicum methanol extracted. On other hand, S. pneumonia and K. pneumonia have the highest minimum inhibitory concentration with the value more than 150 μl/ml O. basilicum ethanol extracted. Fusarium spp. recorded the less MICs on the tested fungi with values of 70, 75 μl/ml O. basilicum methanol and ethanol extracted respectively, while other tested fungi have the same value of MICs 150 μl/ml.
|
|
3.3. Mode of Action: Effect of O. basilicum Oil on Bacterial Cell Wall
- Table 5 detected the values of sugar, protein and phosphorus in Bacillus spp. cell wall, the results showed decreased of the sugar, protein content compared with the normal bacterial cell wall, and that means the O. basilicum methanol extract reduced the bacterial cell wall. Further, the content of phosphorus increased and that may due to the losses of energy components.
|
3.4. Imaging the Cells Using Scanning Electron Microscope (SEM)
- Scanning electron microscope on Figure 3 showed the damage effect of O. basilicum methanol extract on Bacillus spp. cell wall that appears on shrinkage, rupture, and partial deformation.
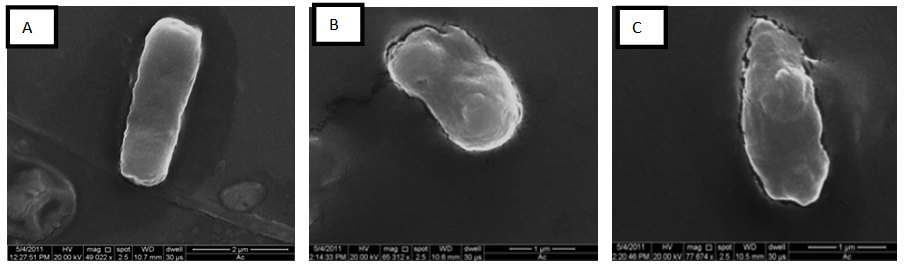 | Figure 3. Scanning electron microscope of Bacillus spp. treated with O. basilicum methanol extract B and C compared with normal cell A |
3.5. Chemical Analysis of Methanol and Ethanol Extract of O. basilicum
- The results of chemical analysis of the methanol extract of O. basilicum were showed in Figures 4 and 5, the results detected presence of ten major components at different ration times. They were as follows: component was 1,6-Octadien-3ol, 3,7-dimethyl. Some of its synonymous names were β-Linalool and linalool, estragole and some of its synonymous names were tarragon and esdragole, linalool, trimrthylsilyl ether, Trimethylsilyl ether glycerol and some of its synonymous names were Glycerol, tris (trimethylsilyl) ether and 2,2,8,8-Tetramethyl, tau.-Cadinol and some of its synonymous names:4-Isopropyl-1,6-dimethyl-1,2,3,4,4a,7,8,8a-octahydro-1-naphthalenol, trans-α-Bergamotene and some of its synonymous names:2,6-Dimethyl-6-(4-methyl-3-pentenyl) bicycle [3.1.1] hept-2-ene, Benzene, 1,2-dimethoxy-4-(2-propenyl0), tetradec anoic acid, trimethylsily ester, α-Linolenic acid,trimetgylsilyl ester and 9, 12 Octadecadienoic (Z, Z)-, trimethylsilyl ester. While the effects of the chemical analysis of O. basilicum ethanolic extract showed the presence of five major components. The detected compounds are: 2-Furanmethanal, 5-ethenyltetrahydro- α, α, 5-trimethyl-,cis-, and some of its synonymous names were cis-Linalool Oxide. And linalool Oxide, 1,6-octadient-3-ol, 3,7-dimethyl-. Some of its synonymous names were β-Linalool and linalool, Bicyclol 2.2.1 lheptan-2-one,1,7,7-trimethyl-(1S)-, and some of its synonymous names were camphor and Levo-(-)-camphor, 3,7-Octadiene-2,6-diol,2,6-dimethyl-, some of its synonymous names were 1,5-Octadiene-3,7-diol, 3,7-dimethyl- and (3E)-2,6-Dimethyl-3,7-octadiene-2,6-diol, and Estargole, some of its synonymous names were tarragon and esdragole.
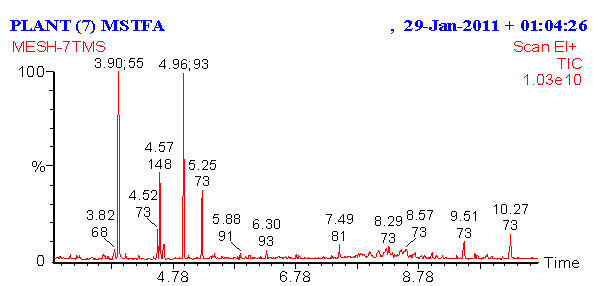 | Figure 4. Chemical analysis of methanol extract of O. basilicum using GC/MS |
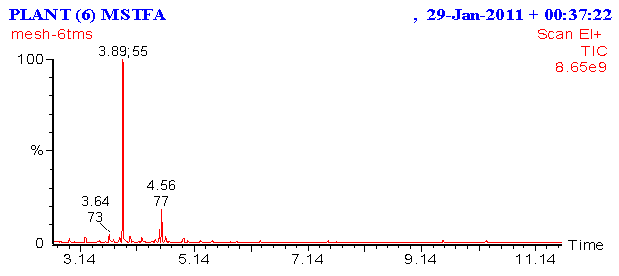 | Figure 5. Chemical analysis of ethanol extract of O. basilicum using GC/MS |
4. Discussion
- The selection of Ocimum basilicum var. Genovese was due to its antimicrobial activities compared to other strains. In the same manner, Carović-Stanko [36] studied essential oils of the three botanical varieties and cultivars of O. basilicum (‘Genovese’, var. purpurascens and var. difforme) as an antibacterial agent. Their results indicated the greatest effectiveness was achieved by the essential oils from O. basilicum ‘Genovese’ which agrees with our study, but the essential oil of O. basilicum var. purpurascens and O. basilicum var. difforme showed the weakest antimicrobial activity.The antimicrobial activities of the plants varied greatly with solvents because there are many factors that influence the active principles present in the plant which include the age of the plant, extracting solvent, method of extraction and time of harvesting plant materials [37, 38].Based on the previously discussed results, the methanol extract has a stronger and broader spectrum of antimicrobial activities compared with the ethanol extracts, thus methanol was recommended for the extraction of the active antibacterial agents from O. basilicum. This result was in accordance with previous studies which reported that methanol was a better solvent for the more consistent extraction of antimicrobial substances from medicinal plants compared to other solvents such as water and ethanol [3, 4, 39]. In contrast, less antimicrobial activity was obtained by [40] who tested the activity of the ethanol extracts of ten plants, including O. basilicum plant, against 14 bacteria, the extracted oil was inactive against all the tested bacteria including S. aureus, Ps. aeruginosa, K. pneumonia and E. coli, but moderate activity was found only against one strain of Ps. aeruginosa. Nedorostova et al. [41] found excellent antimicrobial activity for O. basilicum extract against five foodborne bacteria. Helal et al. [42] noticed that the antimicrobial activity of all tested O. basilicum oils were generally higher against bacteria than fungi and yeast, which confirmed our results.Bokhari [43] tested aqueous and organic extraction (ethyl alcohol, methanol, n-butanol, ethyl acetate or chloroform) of five medicinal plants growing in Jeddah including O. basilicum against species of some dermatophytes and all medicinal plants were active against the six tested dermatophytes. Helal et al. [42] tested the ability of essential oil extracted from five plants including O. basilicum against fungi, bacteria and yeasts in addition to bacteria associated with the contamination of fruit juices. The essential oils under test process showed excellent antimicrobial activity against fungi, bacteria and yeasts. They found that most of the O. basilicum oils inhibit conidia formation of the tested fungi, thus inhibiting fungal growth and flourish. Also, Runyoro et al. [44] found that essential oils of Ocimum species growing in Tanzania were active against three types of yeasts including Candida albicans, C. tropicalis and C. glabrata.According to the survey that was conducted, there are very few studies about the antitumor activity of O. basilicum oils. Basil has shown antioxidant, antimicrobial and antitumor activities due to its phenolic acids and aromatic compounds [45, 46].This work included a study of the mechanism the impact of essential oil of O. basilicum on the bacterial cell wall. Our results indicated that all Gram positive bacteria were more sensitive than Gram negative bacteria. Some studies agree with our findings. The antimicrobial activities O. basilicum against Gram-positive bacteria were higher than Gram-negative bacteria. These observations are likely to be the result of the differences in cell wall structure between Gram-positive and Gram-negative bacteria. Gram-negative has an outer membrane acting as a barrier to many environmental substances including antibiotics [47]. Gram-negative bacteria contain a high level of lipid materials. These materials are thought to make a substantial contribution to the mechanism whereby injurious chemicals are prevented from reaching their sites of action within the cell [48]. Perhaps to be expected, they possess an outer membrane surrounding the cell wall [49] which restricts the diffusion of hydrophobic compounds through its lipopolysaccharide covering.Gas chromatography–mass spectrometry apparatus was used to analyze and define the components of the essential oils which extracted from O. basilicum plant. This is the same method employed by [25, 50], who found that gas chromatography–mass spectrometry (GC–MS) has played the most important role in the identification of the chemical composition of basil essential oils.The data recorded about finding in O. basilicum linalool and camphor is similar to the ones presented by the specific literatures, adding estragole. In a study carried by [51], it was found that inhaling linalool can reduce stress in lab rats. The findings could form the basis of new blood tests for identifying fragrances that can soothe stress. Many scientists have linked basil antimicrobial effects to the presence of high content of linalool.
5. Conclusions
- Searching for new antimicrobial agents from plants and detect its ability to treat diseases caused by resistant microorganisms is needed. Ocimum basilicum L. (Basil) is one of the most common plants used traditionally all over the world and in Saudi Arabia to treat many diseases. Our results of the investigation of this plant showed its ability to be a new source of natural products used as antimicrobial agents.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML