-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2015; 5(3A): 48-54
doi:10.5923/s.materials.201502.08
Vibrational Spectral Studies of Pure and Doped DTGS Crystals
Arun K. J.1, 2, A. K. Batra1, M. D. Aggarwal1, Almuatasim Alomari1
1Department of Physics, Chemistry, and Mathematics (Materials Science Group), Alabama A&M University, Normal, Alabama, USA
2Department of Physics, Sree Kerala Varma College, Thrissur, Kerala, India
Correspondence to: A. K. Batra, Department of Physics, Chemistry, and Mathematics (Materials Science Group), Alabama A&M University, Normal, Alabama, USA.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Single crystals of deuterated triglycine sulfate (DTGS), DTGS doped with deuterated phosphoric acid (DTGS(0.8)P(0.2)) [DTGSP], and L-alanine (DTGS(0.8)P(0.2)—LA (2 gm)) [DTGSP-LA] are grown from deuterated water solution containing glycine, D2SO4, D3PO4, and L-alanine. Vibrational spectroscopic analysis has been under taken to examine the configuration of glycinium ion formation and the changes in the nature of hydrogen bonding due to the partial substitution of SO42- by PO43-. Fourier Transform Infrared (FT-IR) and FT-Raman analysis indicates the presence of both zwitter and glyciniumions in the doped DTGS crystals, which is due to the low incorporation of dopants into the crystal lattice, which supports Hoshino’s theory of spontaneous polarization reversal. Nuclear magnetic resonance studies (1H, 13C, 31P) revealed the possible bond formation during the formation of the compound.
Keywords: Ferroelectric crystal, Infrared spectroscopy, Raman spectroscopy, Nuclear magnetic resonance
Cite this paper: Arun K. J., A. K. Batra, M. D. Aggarwal, Almuatasim Alomari, Vibrational Spectral Studies of Pure and Doped DTGS Crystals, American Journal of Materials Science, Vol. 5 No. 3A, 2015, pp. 48-54. doi: 10.5923/s.materials.201502.08.
Article Outline
1. Introduction
- The TGS crystal family, with the chemical formula (NH2CH2COOH)3•H2SO4, is one of the well-known ferroelectric materials useful for room temperature IR detector applications. Pyroelectric detectors based on TGS are uniformly sensitive to radiations in the wavelength range from ultraviolet to far-IR and do not require cooling as compared to quantum detectors, where low cooling is required. The crystal structure of TGS is monoclinic below Tc (49°C). There are two chemical formulas in the elementary cell of the lattice, and the crystal is monoclinic with the space group P21 in the ferroelectric phase. Above the Tc, in the paraelectric phase, there are two mirror planes at y = 0.25 and 0.75, and space group P21/m. The ferroelectric effect is associated with the asymmetrical arrangement of the glycine group G I versus the mirror plane and the proton exchange between glycine groups G II and G III associated with the exchange role of zwitterions between two groups [1]. The b-cut/(010) crystals are used for detector fabrication, which is also a cleavage plane. Single crystals of TGS are usually grown from an aqueous solution by the method of temperature lowering or solution evaporation [2-7, 9-16]. TGS crystals weighing more than 100 g have also been grown from solution with ethyl alcohol addition [8]. To counter the drawbacks cited above while maintaining the same basic structure, many modifications to TGS have been proposed and evaluated for pyroelectric infrared (PIR) detection applications. These modifications include deuteration of TGS (DTGS). L-alanine has the effect of introducing an internal electric field to produce poled TGS crystals and stabilizing the spontaneous polarization. This is because the alanine molecules possess an extra methyl group that prevents them from rotating within the lattice. The dipole it possesses is thus fixed with respect to the crystal structure and does not disappear at the Tc. The L-alanin LATGS crystals can be thermally cycled through the Tc. They will retain their spontaneous polarization, thereby avoiding the poling process. There is an increase in figure of merit (FOM) depending upon the L-alanine content in the TGS lattice. The uniformity of L-alanine doping in the TGS lattice is also difficult to attain. It can be inferred that deuteration of TGS provides a marked improvement to the RV and also increases the Tc to 60°C, depending on the deuteration level. This is extremely desirable as it increases the temperature range of detector operation. Bye and Keve [6] studied the pyroelectric properties of x-ray irradiated/field-treated TGS detectors and compared these with LATGS detectors. The improvements in FOMs of x-ray/field-treated TGS detectors were comparable to L-alanine-doped TGS detectors. In the recent past, doped TGS crystals with organic molecules have been reported with encouraging results such as L-lysine, urea, urea + L-alanine, thiourea, guanidine, and others [11-16]. A higher performance and increase in range of the operational temperature were offered by TGFB (Triglycine fluoberyllate) and DTGFB. However, the growth and processing of these crystals is dangerous due to the presence of toxic beryllium. Deuterated Triglycine (DTGS) crystal has a Curie temperature, which can be raised to 57-62°C thus the temperature ranges of the devices made of it can also be raised. L-alanine doped deuterated triglycine sulfate (DTGS-LA) crystal creates an inherent bias field due to the doping, preventing the crystal from depolarization, and the pyroelectric property is greatly increased.The pyroelectric figure of merit (FOM = p/ε; p-pyroelectric coefficient, ε-dielectric constant) demands low permittivity and high pyroelectric coefficient. Substitution of glycine with alanine has improved the ferroelectric properties. The amino acids mixed TGS have low permittivity values compared with pure TGS. In the present investigation, deuterated phosphoric acid and the amino acid L-alanine have been substituted in order to improve the pyroelectric figure of merit of DTGSP. The amino acid alanine chosen has a similar structure to glycine. Studies on the advantages of DTGS and doped compounds over TGS as IR detectors have been reported. In the present work, pure and doped DTGS, and DTGSP with L-alanine are grown from solution. We have chosen this study to understand the incorporation of dopant and its bonding nature, with a view of obtaining an insight into the structural aspects.
2. Experimental Procedure
- Deuterated triglycine sulfate (DTGS) was synthesized from the extra pure glycine (99.99%) deuterated sulfuric acid (D2SO4) and deuterated water (D2O) as the solvent according to the reaction3(NH2CH2COOH) + D2SO4 → (NH2CH2COOH)3 D2SO4Deuterated triglycine sulfo phosphate (DTGSP) was obtained by the chemical reaction of glycine, deuterated sulfuric acid, deuterated phosphoric acid, and deuterated water as the solvent with the following chemical composition:3(NH2CH2COOH) + (1-X) D2SO4 + X D3PO4 → (NH2CH2COOH)3 (1-X) D2SO4 X D3PO4where X= 0.2.DTGSP doped with 2 grams of L alanine (DTGSP-LA) is also prepared in the present study.Impurity concentration was minimized by successive recrystallization. The final solution was filtered using 0.1 µm filter paper and kept in a beaker covered with perforated sheets to avoid contamination. After three days of evaporation, solution becomes supersaturated and small crystals were found to grow inside the beaker and are allowed to grow for a maximum possible dimension, and were carefully harvested and subjected to vibrational spectral characterization studies by Fourier transform infra red (FTIR) and FT-Raman analysis, and is also analyzed by nuclear magnetic resonance studies. FTIR spectral analysis in the range of 500-3500 cm-1 was carried out by Thermo Nicolet FTIR spectrometer with a resolution of 8 cm-1 using Globar (ETC Everglo) as the source of IR Radiation. FT-Raman spectra were recorded with a portable Horiba scientific Raman micro spectrometer in the range of 250-2500cm-1 using 785nm laser source. Nuclear magnetic resonance spectra of the grown samples were recorded with Anasazi Eft-60 NMR spectrometer in the 1H, 31P, and 13C modes.
3. Results and Discussion
3.1. FT-IR Spectroscopic Analysis
- Figure 1 depicts the FT-IR spectra of pure DTGS, DTGSP, and DTGSP-LA. In order to understand the existence of dopant and its bonding nature, with a view of obtaining an insight into the structural aspects of increased pyroelectric figure of merit, the present study has been undertaken. The observed wave numbers, relative intensities obtained from the recorded spectra, and the assignments proposed for the pure and doped DTGS crystal are given in table 1.
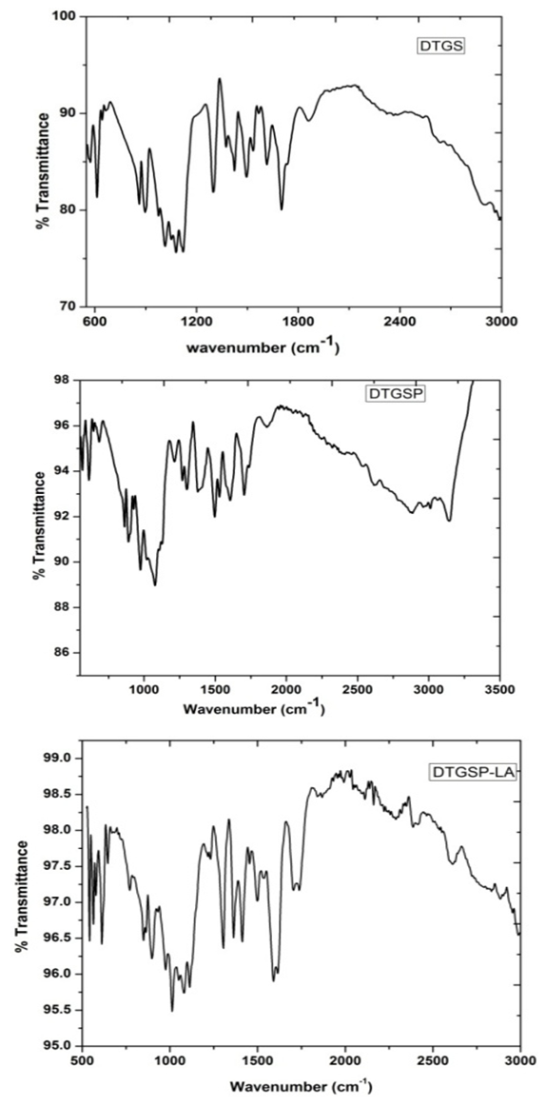 | Figure 1. FTIR Vibrational spectra of pure and doped DTGS crystal |
|
3.2. FT-Raman Spectral Analysis
- Raman spectra of the pure and doped DTGS crystals were recorded in the range from 300-1800 cm-1 is shown in figure 2. By using the spectral data of SO4, PO4, CH2, NH3, C-H, and by comparing the spectra of the different samples, we have assigned the observed lines to the vibrations of the characteristic groups. The vibrational assignments are given in table 2. The low frequency vibrations are assigned to vibration and translation of glycine molecules relative to their linking bonds. The vibrations from 319-400cm-1 are assigned to the vibrations and translations of glycine molecule. The relative intensities of the glycine internal vibration remain almost unchanged, which confirms that doping with D3PO4 and L-alanine does not alter the crystal structure with internal arrangements in the symmetries of groups in the unit cell.
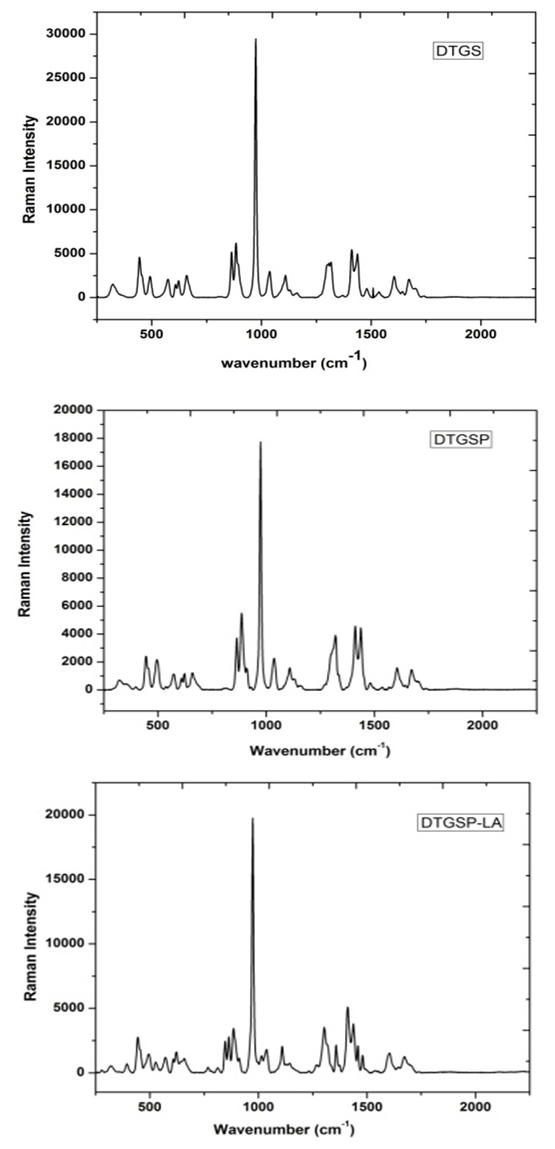 | Figure 2. FT - Raman spectra of pure and doped DTGS crystal |
|
3.3. Nuclear Magnetic Resonance (NMR) Spectra Analysis
- Carbon-13 NMR (13C NMR) is the application of nuclear magnetic resonance spectroscopy to carbon. It is analogous to proton NMR and allows the identification of carbon atoms in an organic molecule just as the proton NMR identifies hydrogen atoms. The 13 C NMR is directly above the carbon skeleton not just the proton to which it is attached. The number of signals tells the number of different carbons, and the splitting signal tells the number of hydrogen attachment, which depends on resolution and concentration due to the low isotopic abundance. In our study, samples are dissolved in deuterated water and 13C NMR spectra are recorded for DTGS, DTGSP and DTGSP-LA, and are shown in figure 3. The peak at 167 ppm found in all the three samples is due to C=O, and the one around 37 ppm found in all the three samples is due to R3-CH bond, wherein the R groups won’t necessarily be simple alkyl groups. The peak at 46ppm confirms C-NH2 and C-CH3 forms a peak at 12 ppm in the sample, which validate our study.
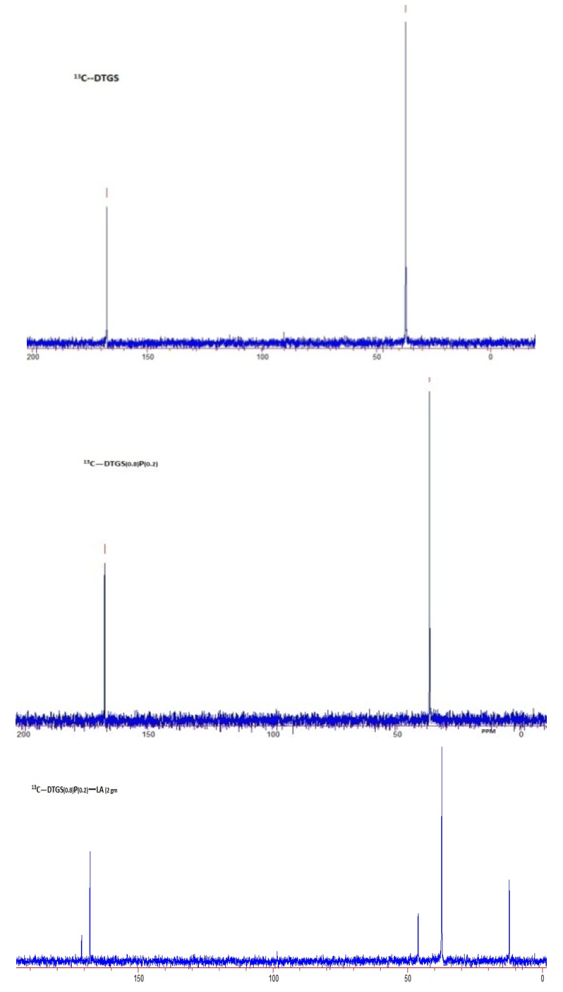 | Figure 3.  NMR spectra of undoped and doped deuterated triglycine sulphate NMR spectra of undoped and doped deuterated triglycine sulphate |
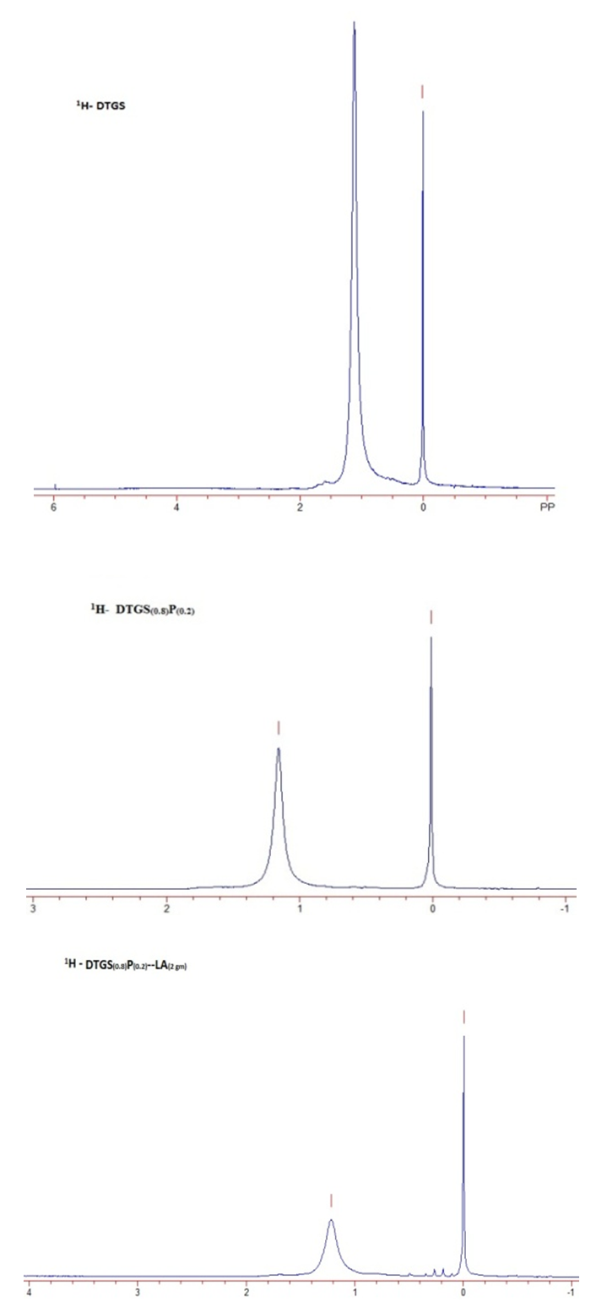 | Figure 4.  NMR spectra of pure and dopant substituted DTGS NMR spectra of pure and dopant substituted DTGS |
 | Figure 5.  NMR spectra of substituted and un-substituted deuterated triglycine sulphate NMR spectra of substituted and un-substituted deuterated triglycine sulphate |
4. Conclusions
- FTIR studies show that in all the spectra there is broadening of the band due to OH stretch of COOH and NH of –NH3+, and some of the bands are either narrowed or broadened in the spectra of the mixed crystals, which is a clear evidence of the presence of amino acid in the doped crystal. Based on the broadening of the peak in FTIR spectrum, we confirm the co-existence of PO43- and L-alanine in DTGS crystal, which does not change the crystal structure of symmetries of groups in the unit cell with a possibility of change in the bond length and bond angles, which in turn constitute a change in the pyroelectric figure of merit compared with TGS. FTIR and FT-Raman studies reveal that no major shift or additional peaks occur in their respective spectra, which rules out the possibility of formation of any other complex compound in alanine doped DTGSP crystal.The spontaneous polarization reversal in TGS is due to the proton transfer between glycine and glycinium ions. This switching mechanism can be confirmed by the existence of a dipolar ion in the unit cell. Vibrational spectra of the samples under the present study indicate the presence of the zwitterions, which supports Hoshino's theory for the spontaneous polarization reversal. NMR spectroscopic analysis confirms the formation different functional group in the compound.
ACKNOWLEDGEMENTS
- The authors wish to thank Brian D. Thomas, chemistry department, AAMU, Normal Alabama, USA for his assistance in the recording of vibrational and NMR spectra of the samples. The author, AKB gratefully acknowledges support for this work through the National Science Foundation grant # EPSCoR R-II-3 (EPS-1158862) and DHS grant # 2010-ST-062-000034. Some work was accomplished with the partial support of NSF APEX project and DUE1238192.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML