-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Materials Science
p-ISSN: 2162-9382 e-ISSN: 2162-8424
2015; 5(2A): 10-15
doi:10.5923/s.materials.201501.03
Ceria Catalyst Promoted with Al3+ and Acidified with PO43- i) Synthesis and Surface Textural Properties
Galal Elmanfe , Saleh M. Bofarwa
Chemistry department, Faculty of Science, Omar Al-Mukhtar University, Al-Bayda, Libya
Correspondence to: Galal Elmanfe , Chemistry department, Faculty of Science, Omar Al-Mukhtar University, Al-Bayda, Libya.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Phosphated ceria were prepared and promoted with different loading levels of aluminum (Al3+); 2, 5 and 10-wt% followed by calcination at 870 K for 3 h. These samples have been characterized by means of thermal gravimetric analysis (TGA), X-ray powder diffraction (XRD), Nitrogen adsorption at 77 K. TGA profiles show phosphated species with different thermal stability in the samples and addition of Al3+ causes an increase in the thermal stability of the surface phosphate species. The obtained results indicated that composites mainly cubic pattern of ceria (fluorite) structure and the addition of Al3+ and/or phosphate has no effect on the bulk structure of ceria but increases the crystallite size. Textural characteristics show that the phosphation and Al-promotion resulted in increased in the surface area. The pore size distribution exhibits a wide spectrum of mesoporosity in all samples.
Keywords: Ceria, Phosphation, Promotion, Texture, Mesoporous
Cite this paper: Galal Elmanfe , Saleh M. Bofarwa , Ceria Catalyst Promoted with Al3+ and Acidified with PO43- i) Synthesis and Surface Textural Properties, American Journal of Materials Science, Vol. 5 No. 2A, 2015, pp. 10-15. doi: 10.5923/s.materials.201501.03.
Article Outline
1. Introduction
- Ceria is widely used in catalytic converters for exhaust gases because of its exceptional redox properties. Ceria able to store oxygen during the lean phase (i.e., excess of oxygen) and to give oxygen back to metal crystallites during the rich phase (when there is virtually no O2 in the gas phase); this is the so-called oxygen storage capacity of ceria. The superficial valence exchange, Ce(IV) - Ce(III), of Cerium makes ceria a good catalyst with high oxygen storage/release capacity and good redox properities. Therefore, ceria based catalysts have been extensively used for fuel reforming e.g., water gas shift reaction, CO preferential oxidation, the synthesis and decomposition of methane, methanol, etc., automotive exhaust cleaning, fuel cell reactions and glass polishing. The surface properties of ceria have been investigated by several authors providing valuable information on surface texture, porosity, redox properties and oxygen mobility in the ceria lattice [1-4]. Ceria is a pale yellow color solid due to oxygen-cerium charge transfer and is known to crystallize in fluorite structure. It is also believed to help in preserving the catalyst surface area, pore size distribution and catalytic activity [5-6]. Pure CeO2 has low thermal resistance and low textural stability, which are not high enough to meet the requirements of high-temperature applications such as three-way catalysts (TWCs) [7, 8]. Hence pure ceria is generally not preferred and the catalytic efficiency may also be reduced at high temperature because of sintering and loss of surface area [9]. This loss in surface area may be attributed to changes in the pore structure and to crystallite growth. Hence it is very much important to improve its textural stability.Ceria possesses versatile acid–base properties, depending, on the nature and temperature of the pretreatment. It may have high number of basic sites of weak or medium strength [10]. Binet et al. also observed that ceria can chemisorb CO or pyridine, but the band positions strongly suggest that the Lewis acidity of ceria is significantly lower than that of zirconia or titania. In contrast to Lewis basicity, the Lewis acidity would decrease upon reduction of ceria. Khalaf [1] found that the addition of ceria onto alumina decrease the activity and selectivity towards propene production from isopropanol decomposition.It was found [11-15] that catalytic activity, thermal stability, and surface area could be significantly improved by adding some additives to certain oxide catalyst surfaces. Phosphates have been claimed to act as a support stabilizer [15-17]. Khalaf et al. [15] also proved no surface area stabilization effect from phosphates in the case of the transition phase (spinel) aluminas. Marcu et al [18] reported that the addition of phosphorus to ceria and increasing its content results in a modification of the physicochemical characteristics of the catalyst, the redox ability of the catalytic material being strongly diminished. At the same time, by adding phosphorus to ceria and increasing the phosphorus content, a decrease of the catalytic activity accompanied by an important increase of the selectivity for isobutene, mainly at the expense of carbon oxides, was observed. A compensation effect in catalysis was also observed for the isobutane conversion on this series of catalysts. El-Idrissi et al. [14] showed that the addition of phosphorus to Cr/TiO2 resulted in a remarkable increase in the activity and ethylene selectivity. They attributed these improvements to the fact that phosphorus contributes to the stabilizing octahedral Cr3+ species in a well-defined environment and adjusts in an advantageous way the acid properties of the active surface.The promoting effects of metal oxides can act as textural modifier and chemical promoting. Thus, there are several CeO2-based systems such as ceria-zirconia, ceria-alumina, ceria-alumina modified with copper, ceria-silica and ceria modified paladium have been studied for their catalytic properties [19-22]. Recently efforts have also been made to synthesize nanocrystallites of ceria having better physicochemical properties for diverse applications. But there are not many studies reported in the literature in this direction and the role of promotion with metals is yet not totally understood. The present article provides an overview of some structural and textural aspects of ceria acidified with phosphorus and promoted with Al.
2. Experimental
2.1. Materials
- Cerium oxide was prepared by precipitation method as in literatures [1, 15]. In brief, precipitation of ceria was carried out by slowly adding 1 M aqueous solution of cerium (III) nitrate hexahydrate (Ce(NO3)·6H2O) (Sigma–Aldrich, puriss; ≥99.0%) into a well-stirred precipitating solution of ammonium hydroxide (Sigma–Aldrich, ACS reagent,- 30.0% NH3 basis). The pH of the solution was carefully controlled to reside at 11. The synthesis time for stirring the solution was 48 h. The resulting precipitate was filtrated, washed with deionized water, dried overnight at 373 K, to give cerium hydroxide, and calcined at 873 K for 3 h in air, to give cerium oxide.Aluminium hydroxide prepared as in the literature [15] by precipitation method from a 0.3M aqueous solution of Al(NO3)3. 9H2O and ammonium hydroxide at pH=8.Ceria promoted Al(III) samples have been prepared by impregnation method by adding the needed amount of aluminium hydroxide (2, 5 and 10 %Al by weight) to appropriate amount of cerium hydroxide. The impregnation solution was stirred 1 h, then dried at 373 K overnight.Phosphation process; the dried samples of ceria promoted with Al(III) was added to 50 ml from the appropriate amount of diammonium hydrogen phosphate (6%PO43- by weight) and stirred for 1 h, dried at 373 K overneight, followed by calcination at 873 K for 3h. The resulting samples were denoted as xPAlCe, x is equal 2, 5 and 10 which is the loading levels of Al.
2.2. Apparatus and Techniques
2.2.1. Thermal Analysis
- Thermogravimetric analysis (TGA) and deferential thermal analysis (DTA) were performed between room temperature and 1273 K in a static atmosphere of air, using Linseis STA PT 1600 thermogravimetric analyzer. The rate of heating was standardized at 10 K /min. and small portions (5-15 mg) of the sample were used in TG measurements.
2.2.2. X-ray Powder Diffractometry
- XRD diffractograms were recorded for all samples using a model JSX-60PA JEOL diffractometer (Japan) using Cu Kα radiation (λ=1.5418 Å). The generator was operated at 35 kV and 20 mA. The samples were scanned in the range of 2θ = 10–70° at a scanning speed of 6 ° min-1. For identification purposes, diffraction patterns (I/Iº) versus d spacing (Å) were matched with the relevant ASTM standards [23]. The crystallite size D of the samples was calculated using the Scherrer relationship [24].
2.2.3. Nitrogen Adsorption Isotherm Measurement
- Full nitrogen adsorption/desorption isotherms at 77 K were obtained using a NOVA 2200, version 6.10 high-speed gas sorption analyzer (Quantachrome Corporation USA). The calcined samples were first degassed at 470 K for 1 h. Twenty four-point adsorption and desorption isotherms were obtained, from which BET surface areas were derived using standard and well-established methods [25, 26].
2.2.4. Potentiometric Titration
- The total acidity of the solid samples under investigation was measured by means of potentiometric titration [27, 28]. The solid catalyst (0.1 g) was suspended in 10 ml acetonitrile (Merck), and agitated for 4 h. Then, the suspension was titrated with 0.1 N n-butylamine in acetonitrile at 0.10 ml min-1. The electrode potential (E) variation was measured with SevenMulti, Mettler-Toledo GmbH, Switerland. To explain the obtained results, it was suggested that the initial electrode potential (Ei) indicates the maximum acid strength of the sites. Cid and Pecci [29] scale of acid strength measurement, defined as follows: Ei > 100 mV for very strong sites; Ei = 0–100 mV for strong acid sites; Ei = -100 – 0 mV weak sites; and finally Ei < -100 mV for very weak sites.
3. Results and Discussions
3.1. Thermal Analysis
- Figure 1 shows the thermal behaviour of pure precursor, phosphated and Al(III)-promoted phosphated ceria samples heated up to 1273 K. The mass loss in the low temperature range (room temperature to 450 K) was due to the removal of adsorbed water (6-10% mass loss) for the pure and modified ceria. The mass loss (ca 65%) from 450 to 510 K temperature was attributed to the decomposition of precursors (ceria and alumina gels). Another mass loss, at before 800 K, near to 77% at this temperature corresponds to the crystallization of ceria nanocrystallites.
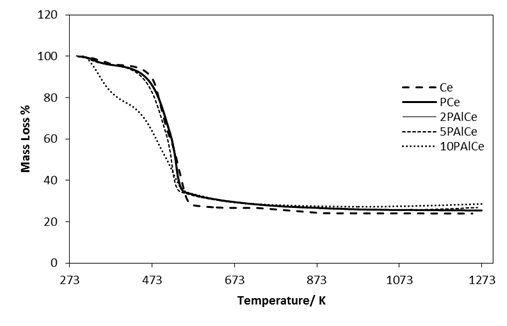 | Figure 1. TGA for pure and modified ceria samples |
3.2. X-ray Diffraction (XRD)
- XRD patterns for pure precursor and modified ceria samples were shown in Figure 2. These diffractograms indicate that for pure ceria, the XRD difractogram displayed sharp and intense peaks corresponding to cubic CeO2 fluorite structure as matched with the database in JCPDS (file number 04-0593) [30]. The modification of ceria with phosphate ion has no effect on ceria crystal phase (fluorite). The promotion with Al(III) has slightly effect, especially on high loading levels, on the crystal phase of ceria. Only there is a decrease in the crystallization degree by increasing the loading levels of Al(III), as shown from the peaks height and decrease in crystallite size. These results are similar to those previously reported [1, 31]. The γ-Al2O3 structure (ASTM card No. 29-1486) [15] was slightly noticed in higher loading level (10%) than other samples. The crystallite size was estimated from the Scherrer equation [24], and the results are cited in Table 1. The smallest crystallite size (36.0 nm) for the present samples was detected for the 10PAlCe. For other samples, the crystallite sizes were estimated as 44.4, 36.2, 42.8, 39 and 36.2nm for Ce, PCe, 2PAlCe and 5PAlCe respectively. It is clear that, there is a slightly decrease in the crystallite size by increasing Al(III) loading levels. These data are agreed with that obtained from nitrogen sorption isotherms.
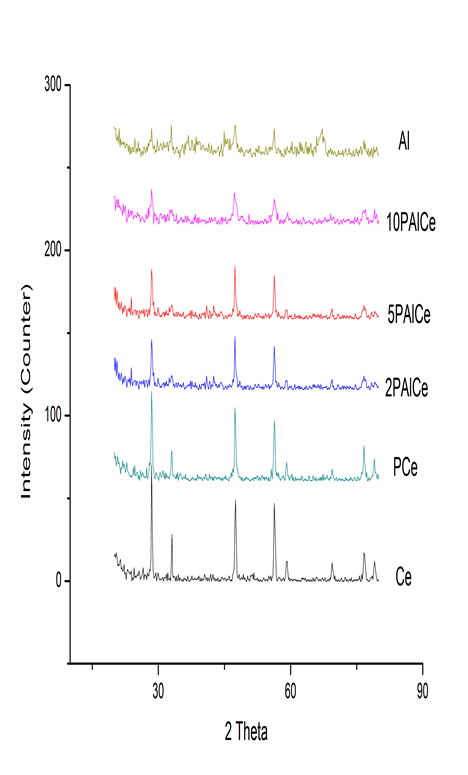 | Figure 2. XRD for pure and modified ceria samples as well as Al2O3, (Al) |
|
3.3. Surface Texture
- The different surface characteristics for pure and modified ceria catalysts were determined from low temperature nitrogen adsorption isotherms conducted at 77 K. These characteristics included specific surface area (SBET), total pore volume (Vp) and average pore radius (rH), the data included in (Table 1). Nitrogen adsorption/desorption isotherms (Fig. 3) for the studied catalysts are of type IV based on Brunauers classification [32] and exhibiting very reduced H2 hysteresis loop according to International Union of Pure and Applied Chemistry (IUPAC) classification, indicating that the sample possesses mesoporous structure and ink-bottle-like pores of varying radius [33]. All the samples have a close closure point at P/Po = 0.4. This may actually mean that the complete monolayer formation takes place slowly and there is an effective contribution of micropores to the adsorption on the samples. This is confirmed by the pore size distribution (PSD) curves that were obtained from the desorption isotherms; see Figure 4. It also means that the capillary condensation might start from the pore size at about 3 nm.
 | Figure 3. N2-sorption isotherms for pure and modified ceria |
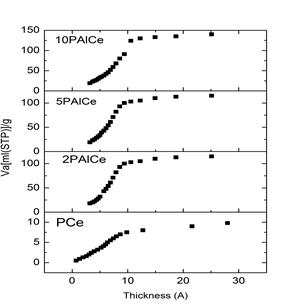 | Figure 4. t-Plots for and modified ceria |
 | Figure 5. Pore size distribution curves for pure and modified ceria |
3.4. Acidity Measurement
- For the catalysts under investigation, the total number of acid sites and their relative strengths can be measured by means of potentiometric titration with 0.1N n-butylamine. It was suggested that the initial electrode potential (Ei) refers to the maximum acid strength of the sites. The value of meq amine/g solid, where the plateau is reached in titration curves (Fig. 6), indicates the total number of acid sites [38]. Table 2 shows the potentiometric titration results for all samples. From these results, one can concluded that both pure ceria and alumina samples have weak acid sites and its maximum strength is equal -80 and -65mV, respectively. The addition of phosphate causes an increase in its acidic strength, but remains in weak range, to become -40 and -22 mV for the samples PCe and PAl respectively. The addition of phosphate after promotion with Al(III) causes enhancement of the acidic strength in the range of weak acid sites.
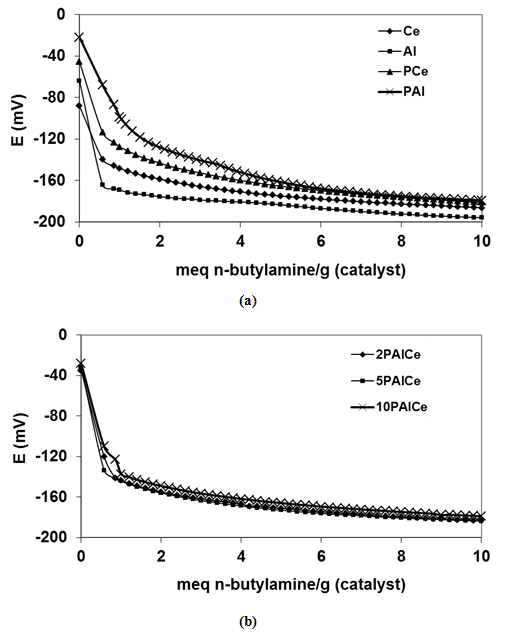 | Figure 6. Potentiometric titration curves for (a) pure alumina, ceria and phosphated samples and (b) phosphahted ceria promoted with Al(III). E is the electrode potential (mV) |
4. Conclusions
- From this study, one can noticed the following conclusions: a) the addition of phosphate to pure ceria can stabilize the specific surface area, modify the porosity and increase the acidity of the catalysts, b) The promotion with Al(III) causes a modification in surface area and porosity, in addition, increasing the acidity of the samples.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML