-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Instrumentation Science
p-ISSN: 2324-9994 e-ISSN: 2324-9986
2013; 2(A): 8-14
doi:10.5923/s.instrument.201306.02
Extractive Colourimetric Determination of Pipazethate HCl by Ion-pair Complex Formation in Pure Form, Dosage Form, Urine and in Presence of its Degradation Products
Mervat M. Hosny
Analytical chemistry department, Faculty of Pharmacy, Zagazig University, Zagazig, 44519, Egypt
Correspondence to: Mervat M. Hosny, Analytical chemistry department, Faculty of Pharmacy, Zagazig University, Zagazig, 44519, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
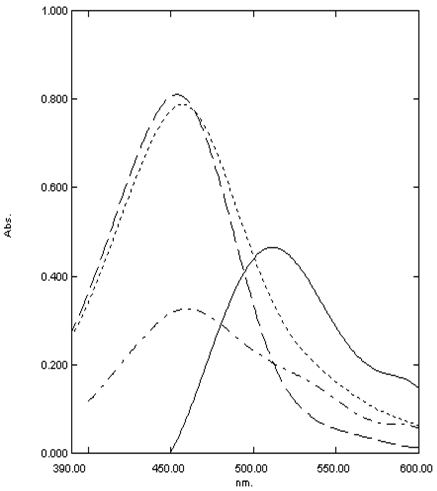
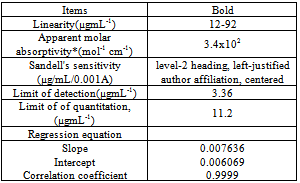
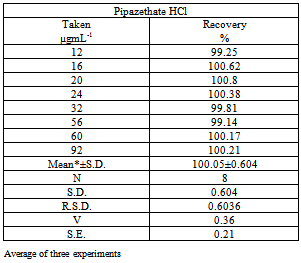
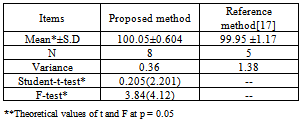
A simple ,sensitive and accurate spectrophotometric method based on extractable ion-pair formation of pipazethate HCl with chromazurol S., exhibits an absorption maximum at 512 nm, was described. Beer’s law was obeyed over the concentration range of 12 – 92 µg mL-1, apparent molar absorpitivity, limit of detection (LOD) and limit of quantitation. (LOQ) were 3.4x102 mol-1L cm-1, 3.36 and 11.2 µg mL-1, respectively. Precision of results, expressed as intra-day and inter-day relative standard deviation values, are satisfactory. The method was successfully applied for the determination of pipazethate HCl in dosage forms and in spiked human urine, no interference was observed from common pharmaceutical additives. Statistical comparison of the results with reference method showed an excellent agreement and indicated no significant difference in precision. Pipazethate HCl was subjected to the stress conditions of acidic, basic, oxidative, hydrolytic, thermal and photolytic degradations. The method retain its accuracy in the presence of basic, acidic and oxidative degradants.because basic degradants give no reaction with chromazurol S while both acidic and oxidative degradants gave yellow colour which was measured at 453nm and 457 nm respectively.
Keywords: Pipazethate, Human Urine,Degradation Products
Cite this paper: Mervat M. Hosny, Extractive Colourimetric Determination of Pipazethate HCl by Ion-pair Complex Formation in Pure Form, Dosage Form, Urine and in Presence of its Degradation Products, International Journal of Instrumentation Science, Vol. 2 No. A, 2013, pp. 8-14. doi: 10.5923/s.instrument.201306.02.
Article Outline
1. Introduction
- Pipazethate HCl (2-(2-piperidinoethoxy)ethyl 10H-pyrido [3,2-b][1,4]benzothiadiazine-10-carboxylate hydrochloride is a non narcotic antitussive and cough suppressant drug that acts centrally[1] it also has some peripheral effects on non-productive cough Figure 1. The onset of action takes about 10–20 min and the therapeutic action lasts for 4–6 h[1-3].
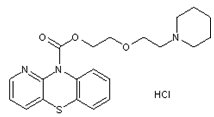 | Figure 1. Chemical structure o fpipazethate HCl |
2. Experimental
2.1. Instrumentation
- A Shimatzu UV and visible recording spectrophotometer (UV 260) with matched 10-20 quartz cells was employed for all absorbance measurements.
2.2. Chemicals and Reagents
- All of the chemicals used were of analytical or pharmaceutical grade and used without further purification. Double distilled water was used to prepare all solutions.Pipazethate-HCl was provided by Egyptian International Pharmaceutical Industries Co. (EPICO) (Cairo, Egypt). Selegon® 20 mg tablets was purchased from the local market. Chromazurol S, 0.3% w/v and 5 10-4 M aqueous solutions by dissolving 0.3 and 0.0303 gm in 100 ml distilled water. Methylene chloride solvent (Wako pure chemical company, Ltd.).Sodium hydroxide, hydrochloric acid, hydrogen peroxide were purchased from Sigma–Aldrich (St. Louis, MO, USA).Urine: Sample was collected from healthy volunteers and kept frozen until use after gentle thawing.Standard solutions 0.4 mg/mL solutions of pipazethate HCl was prepared by dissolving 10 mg of the drugs in 25 mL distilled water.
2.3. General Recommended Procedures
2.3.1. Procedure for Calibration Curve
- Aliquots (0.3-2.3 mL) of standard solution (0.4 mg/mL) pipazethate HCl in the concentration range of (12-92µgmL-1) were transferred into a series of 50 mL separating funnels. 1 mL dye was added , the volume of the aqueous phase was adjusted to 10 mL with distilled water and mixed well. The funnels were shaken vigorously with 10 mL methylene chloride for 2 minute.The two phases were allowed to stand for clear separation.Anhydrous sodium sulphate was used to dehydrate the organic layer.The absorbance of the organic layer was measured at 512nm against blank.
2.3.2. Procedure for the Assay of Tablets
- Twenty Selegon® tablets, each labeled to contain 20 mg Pipazethate HCl were accurately weighed, finally powdered and mixed well., specific quantity of powdered tablets equivalent to 10 mg pure drug were dissolved in distilled water in 25 mL volumetric flask , solutions were filtered and completed to volume with distilled water. Procedures was completed as in general procedures applying standard addition technique.
2.3.3. Procedures of Different Degradation Products
- Pure active drug was stressed under different stress conditions to establish a stability indicating method.A. Procedure for degradation in solutions:20% methanol was used as a co-solvent to avoid any precipitation. Solutions for alkali, acidic, oxidative and neutral degradation studies were prepared as follow: a 5 mg of Pipazethate-HCl was dissolved in 10 ml of methanol-and 40 ml of either 2M NaOH, 2M HCl, 20% H2O2 or water respectively, solutions were protected from light and exposed to dry heat (80ºC) in an oven for 8.0 hours[11], then The absolute recovery was determined for pipazethate HCl by comparing the representative absorbance of stressed sample and that of the pure drug at the same concentration.B. Degradation in solid form:For temperature stress studies; a 5 mg of P-HCl powder was protected from light and exposed to dry heat at 80ºC for 8.0 hours, the powder was dissolved in 50 ml distilled water. For photostability studies; a 100 mg of Pipazethate HCl powder was spread on a glass dish (less than 2mm thickness) and exposed to direct day light for 4 hours,[11] the powder was dissolved in 50 ml distilled water, then the absolute recovery was determined for pipazethate HCl by comparing the representative absorbance of the stressed sample and that of the pure drug at the same concentration.
2.3.4. Procedure for the Assay of Urine Sample
- The proposed method was applied for the determination of pipazethate HCl in human urine ,to prepare spiked urine sample the collected human urine was mixed then human urine was (1:1) diluted with distilled water .For the determination of pipazethate HCl in urine 1.0 mL of the diluted human urine was put in 50 mL separating funnel then the solution was prepared and then absorbance was measured following the same procedure as that of standard solutions. The absolute recovery was determined for pipazethate HCl by comparing the representative absorbance of treated urine sample and that of the pure drug at the same concentration.
2.3.5. Determination of Stoichiometry of the Reaction
- A. Determination of stoichiometry of the reaction (Job's method of continuous variation)[15]:A series of standard equimolecular 5 10-4 M solutions of the drug (vd) and chromazurol S (vr) in different complementary volumes totaling 10 ml were transferred into 50 ml separating funnels, procedure completed as general procedure and the absorbance was measured at the specified λmax.B. Determination of stoichiometry of the reaction (Molar ratio method[16]:Equimolar (5×10-4M) solution of chromazurol S was added to fixed aliquots of drug solution (Vd). Absorbance was measured at the specific λmax.
3. Results and Discussion
- Ion pair spectrophotometric method involves the formation of ion-association coloured complex between drug and dye followed by extraction with organic solvent. or using surfactant without extraction procedure. Many drugs are easy to be determined by spectrophotometry based on ion-association complexe formation either in pure forms or in biological fluids[17-19].The principle of this method based on allowing the drug which has basic cationic center to react with the anionic center in the dye to form coloured ion-pair complexes extractable with methylene chloride then measured at specified λmax (Figure 2).
3.1. Optimization of the Reaction Conditions
- The effect of essential parameters was described.
3.1.1. Effect of Reagent Volume
- Various volumes of reagent were added to fixed concentrations of pipazethate HCL, 1.0mL of o.3% chromazurol S solution was found to be sufficient for the production of maximum and reproducible colour intensity. Higher concentration of the reagent did not increase the absorbance and colour intensity of the formed ion – associate.(Table 1)
3.1.2. Effect of Solvent
- The most convenient solvent for the formed ion associates which exhibit the maximum absorbance, high extraction power and stable colours is methylene chloride. In all cases the aqueous to organic phase ratio of 1:1 was the most suitable for the ion-associate extraction. Complete extraction was attained by using single portion of 10 mL solvent.
3.1.3. Effect of pH
- To examined the effect of pH value for the ion-associates formed between pipazethate hydrochloride and chromazurol S different types of buffer were used.(at pH range from 2.1-10) The ion associate formed without buffer gave the highest absorbance values
3.1.4. Effect of Reaction Time
- The reaction time required for complete colour development of ion-pair complex was studied. It was found that maximum absorbance was attained immediately.The ion-pair formed was stable for at least 30 minutes.
3.1.5. Effect of Surfactant
- Using various dispersing agents such as sodium lauryl sulphate, methylcellulose, tween40 investigated that tween 40 and sodium lauryl sulphate give no results while using methylcellulose give law absorbance than extractive method so methylene chloride was used as extractive organic solvent.(Table 1)
3.2. Stoichiometric Ratio
- In order to investigate the molecular ratio of the complexe formed between pipazethate HCL and the reagent at the selected conditions, the molar ratio and continuous variation methods were carried out. Results indicated that the molar ratio of the drugs to reagent was found to be (3: 2) in all ion – associate formed. In continuous variation method, 5.0 × 10−4 mol L–1 solutions of drug and dye stuff were mixed in varying volume ratio in such a way that the total volume of each mixture was the same. The absorbance of each solution was measured and plotted against the mole fraction of the drug. Figures (3,4) Under the experimental conditions described above the optical characteristics such as Beer’s law limits, Sandell’s sensitivity and molar absorptivity were calculated for the proposed methods and the results are summarized in Table 1.
|
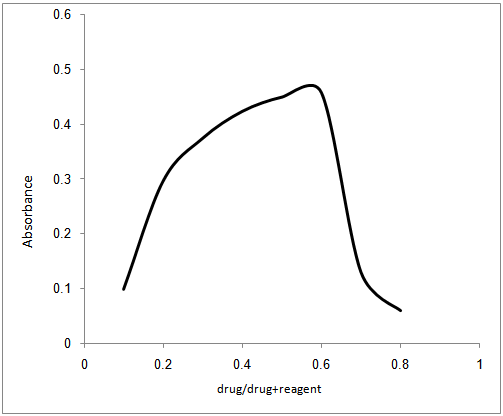 | Figure 3. Molar ratio plot of the reaction between 5x10-4M pipazethate HCl and 5x10-4M chromazurol S |
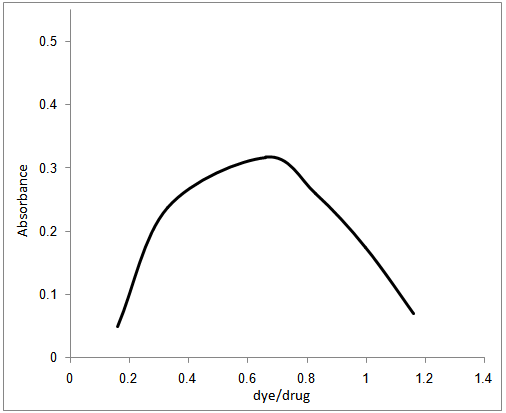 | Figure 4. Mole-ratio plot for (5x10-4M)pipazethate HCl and (5x10-4M) chromazurol S |
|
3.3. Dosage Forms Analysis
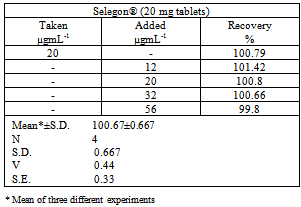
- The proposed method was applied for the determination of Pipazethate HCl in commercially available Selegon® tablets using standard addition procedure (table 3.)InterferencesExperiments showed that there was no interference from additives and expedients, e.g. hydroxyl propylmethylcellulose, calcium phosphate dibasic anhydrouslactose, glucose, fructose, and starch for the examined methods.
|
3.4.Validation
- The proposed method was validated according to the International Conference on Harmonization (ICH) guidelines[20.]
3.4.1. Linearity
- Calibration curve test solutions were prepared from pipazethate stock solution at the concentration range of (12-92µgmL-1) in triplicate. Linearity was established by the regression equation(equation 1)
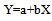 | (1) |
|
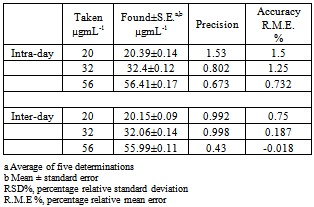
3.4.2. Precision and Accuracy
- The assays described under “general procedures were repeated five times within the day to rmine the repeatability (intra-day precision) and five times on different days to determine the intermediate precision (inter-day precision) of the methods.These assays were performed for three levels of analyte. The results of this study are summarized in Table 5. The percentage relative standard deviation (%R.S.D.) indicating high precision of the method. Accuracy was evaluated as percentage relative mean error (R.M.E) between the measured mean concentrations and taken concentrations for pipazethate hydrochloride {bias % =[(Concentration found - known concentration) x 100/known concentration]} was calculated at each concentration. Percent relative mean error (%R.M.E) values demonstrate the high accuracy of the proposed method.
3.4.3. Specificity
- Specificity is the ability of the analytical method to differentiate between pure drug and other components that may be present. The specificity of the proposed method was determined by giving different results when the degradation products of the drug under various stress conditions undergo reaction with the same reagents.
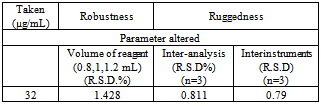
3.4.4. Robustness
- The robustness of the method was evaluated by making small incremental changes in the volume of reagent and the effect of the changes was studied by calculating the mean R.S.D values. The changes had negligible influence on the results as revealed by small intermediate precision values expressed as% R.S.D (table 6)
3.4.5. Ruggedness
- Method ruggedness was expressed as the R.S.D of the same procedure applied by three different analysts as well as using three different instruments. The inter-analysts R.S.D were calculated. The results are shown in Table 6. suggesting that the developed methods were rugged.
|
|
3.5. Degradation Behavior of Pipathezate Hydrochloride
- Using of a 20% methanol as a co-solvent was found to be very convenient to avoid precipitation and also to avoid many time consuming steps such as filtration, washing, drying and reconstitution of degradation products; other degradation products may be formed during multiple preparation steps of the main degradation products and lead to misjudging, as the main degradation products and their stability are unknown in most cases11. In this work; degradation products were studied to measure the selectivity of the proposed method.
3.5.1. Alkaline Conditions
- It was observed that the alkaline degradation product of pipazethate HCl gave no result with chromazurol S ,that is mean that assay of pipazethate HCl can be done in presence of its alkaline degredation product.
3.5.2. Acidic Conditions
- Acidic degradation of Pipazethate HCl showed a yellowish orange colour after extraction of the product of its reaction with chromazurol S which gave maximum absorbance at 453 nm. (Figure 1)
3.5.3. Oxidative Conditions
- Pipazethate hydrochloride was found to be liable also to oxidative degradation; then when this oxidative product react with chromazurol S it gave a yellowish orange colour after extraction which can be measured at 457 nm.(Figure 1)
3.5.4. Neutral Conditions
- Pipazethate HCl was found to be more stable under neutral conditions., a small Temperature stress and photo-stability studies .The product of the drug after exposed to the previous conditions then proceed to the proposed reaction gave results with good recoveriesIn conclusion pipazethate HCl can be measured in presence of its alkaline, acidic and oxidative degradation products.
3.6. Application to Spiked Human Urine
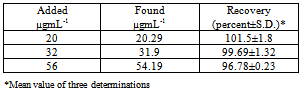
- As another application of the studied method, recovery from human urine samples was carried out and treatment of drug with urine without any extraction step. Recovery studies were performed with the sample containing various amounts of pipazethate HCl. The results of recovery studies (Table 7.) revealed that, there was no interference from other constituents present in the urine in the method. The mean percent recovery obtained from five replicate measurements of urine containing pipazethate HCl ind indicate that the proposed method was effective for the determination of the drug in urine samples.
|
4. Conclusions
- The proposed method is simple, rapid, accurate and precise, The reagent utilized in the proposed methode is cheap, readily availableThe proposed method does not involve any critical reaction conditions or tedious sample preparation.The method is unaffected by slight variations in the experimental conditions, such as reagent concentration .The proposed method can be used for analysis of pipazethate hydrochloride in pharmaceuticals and in spiked human although there is a previous study for the determination of pipazethate hydrochloride in presence of its degradation product using HPLC we try in our study to establish a spectrophotometric method for the analysis of the drug in the presence of its degradation product as the spectrophotometric method is more simple , not complicated , there is no need for expensive solvent ,do not require any expensive equipment and specialized technicians when compared with HPLC, chemiluminescence, and bioassay techniquesThe method is free from interference by common additives and excipients.The comparative study of the molar absorptivity indicated good sensitivity of the proposed method.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML