| [1] | F. Hoffmann, M. Cornelius, J. Morell, M. Froeba, Silica-Based Mesoporous Organic–Inorganic Hybrid Materials, Angew Chem Int. 45, 3216-3251, 2006. |
| [2] | Y. Wan, D. Zhao, On the Controllable Soft-Templating Approach to Mesoporous Silicates, Chem. Rev., 107, 2821, 2007. |
| [3] | C. Sanchez, C. Boissiere, D. Grosso, C. Laberty, L. Nicole Design, synthesis, and properties of inorganic and hybrid thin films having periodically organized nanoporosity, Chem. Mater. 20, 682, 2008. |
| [4] | C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli, J. S. Beck, Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism, Nature, 359, 710, 1992. |
| [5] | J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olson, E. W. Sheppard, A new family of mesoporous molecular sieves prepared with liquid crystal templates, J. Am. Chem. Soc., 114, 10834, 1992. |
| [6] | A. Stein, B. J. Melde, R. C. Schroden, Hybrid Inorganic Organic Mesoporous Silicates-Nanoscopic Reactors Coming of Age, Adv. Mater., 12, 1403, 2000. |
| [7] | A. Sayari, S. Hamoudi, Periodic Mesoporous Silica-Based Organic−Inorganic Nanocomposite Materials, Chem. Mater., 13, 3151, 2001 |
| [8] | B. Naik, N. Ghosh, A Review on Chemical Methodologies for Preparation of Mesoporous Silica and Alumina Based Materials, Recent Patents on Nanotechnology, 3[3], 213-224, 2009. |
| [9] | M. A. Wahab, II. Kim, C. Ha, Hybrid periodic mesoporous organosilica materials prepared from1,2-bis(triethoxysilyl)ethane and (3-cyanopropyl)triethoxysilane, Microporous and Mesoporous Materials, 69, 19–27, 2004. |
| [10] | S. Inagaki, S. Guan, Y. Fukushima, T. Ohsuna, O. Terasaki, Novel Mesoporous Materials with a Uniform Distribution of Organic Groups and Inorganic Oxide in Their Frameworks, J. Am. Chem. Soc., 121, 9611–9614, 1999. |
| [11] | B. J. Melde, B. T. Holland, C. F. Blanford, A. Stein, Mesoporous Sieves with Unified Hybrid Inorganic/ Organic Frameworks, Chem. Mater., 11, 3302–3308, 1999. |
| [12] | T. Asefa, M. J. MacLachlan, N. Coombs, G. A. Ozin, Periodic mesoporous organosilicas with organic groups inside the channel walls, Nature, 402, 867–871, 1999. |
| [13] | Frank Hoffmann, Michael Fröba, Vitalising porous inorganic silica networks with organic functions—PMOs and related hybrid materials, Chem. Soc. Rev., 40, 608–620, 2011 |
| [14] | X. Wang, Y. H. Tseng, J. C. C. Chan, S. Cheng, Catalytic applications of aminopropylated mesoporous silica prepared by a template-free route in flavanones synthesis, J. Catal., 233, 266, 2005. |
| [15] | C. Lei, Y. Shin, J. Liu, E. J. Ackerman, Entrapping enzyme in a functionalized nanoporous support, J. Am. Chem. Soc., 124, 11242, 2002. |
| [16] | B. Munoz, A. Ramila, J. Perez-Pariente, I. Diaz, M. Vallet-Regi, MCM-41 Organic Modificationas Drug Delivery Rate regulator ,Chem. Mater., 15, 500, 2003. |
| [17] | A. Walcarius, M. Etienne, B Lebeau, Rate of access to the binding sites in organically modified silicates. 2. Ordered mesoporous silicas grafted with amine or thiol groups, Chem. Mater., 15, 2161, 2003. |
| [18] | A. M. Lui, K. Hidajat, S. Kawi, D. Y. Zhao, A new class of hybrid mesoporous materials with functionalized organic monolayers for selective adsorption of heavy metal ions, Chem. Commun., 13, 1145, 2000. |
| [19] | M. C. Burleigh, M.A. Markowitz, M. S. Spector, B. P. Gaber, Amine-Functionalized Periodic Mesoporous Organosilicas, Chem. Mater.,13, 4760-4766, 2001. |
| [20] | N. Hao, Z. Wu, P. A. Webley, D. Zhao, Synthesis of ordered mesostructured polymer–organosilica composites by the triconstituent co-assembly method, Materials Letters, 65, 624–627, 2011. |
| [21] | A. Katiyar, S. Yadav, P. G. Smirniotis, N. G. Pinto, Synthesis of ordered large pore SBA-15 spherical particles for adsorption of biomolecules, Journal of Chromatography A, 1122, 13–20, 2006. |
| [22] | M A. Wahab, I. Kim, C. Ha, “Bridged amine-functionalized mesoporous organosilicamaterials from1,2-bis(triethoxysilyl)ethane and Bis[(3-trimethoxysilyl)propyl] amine, Journal of Solid State Chemistry, 177, 3439–3447, 2004. |
| [23] | W. J. J. Stevens, K. Lebeau, M. Mertens, G. V. Tendeloo, P. Cool, E. F. Vansant, Investigation of the Morphology of the Mesoporous SBA-16 and SBA-15 Materials, J. Phys. Chem. B, 110, 9183-9187, 2006. |
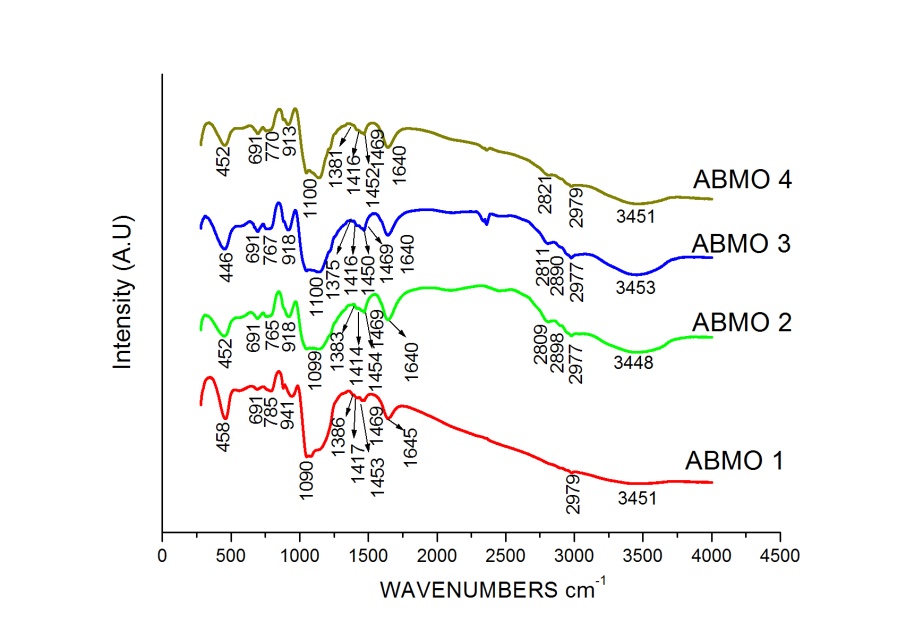
| [24] | R. P. Alonso, F. Rubio, J. Rubio, J.L. Oteo, Characterisation of the pyrolysis process of boron-containing ormosils by FT-IR analysis, J. Anal. Appl. Pyrolysis, 71, 827–845, 2004. |
| [25] | D. Berto, M, Giania, P. Taddei, G. Bottura, Spectroscopic evidence of the marine origin of mucilages in the Northern Adriatic Sea, Science of the Total Environment, 353, 247– 257, 2005. |
| [26] | C. Estournès, T. Lutz, J. Happich, T. Quaranta, P. Wissler, J.L. Guille, Nickel nanoparticles in silica gel: preparation and magnetic properties, Journal of Magnetism and Magnetic Materials, 173, 83- 92, 1997. |
| [27] | K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A Pierotti, J. Rouqeurol, T. Siemieniewska, Reporting physisorption data for gas/solid systems with Special Reference to the Determination of Surface Area and Porosity, Pure& Appl. Chem. 57, 603-619, 1985. |
| [28] | S. Choi, H. Im, J. Kim, The thermal conductivity of embedded nano-aluminum nitride-doped multi-walled carbon nanotubes in epoxy composites containing micro-aluminum nitride particles, Nanotechnology, 23[6], 065303, 2012. |
| [29] | I. Denev, I. Markova, FT-IR spectroscopy investigations of Co nanostructured materials, Journal of the University of Chemical Technology and Metallurgy, 43[4], 427-432, 2008. |
| [30] | S. Shylesh, R. K. Jha, A. P. Singh, Assembly of hydrothermally stable ethane-bridged periodic mesoporous organosilicas with spherical and wormlike structures, Microporous and Mesoporous Materials, 94, 364–370, 2006. |
| [31] | S. Shylesh, Ch. Srilakshmi, A. P. Singh, B.G. Anderson, One step synthesis of chromium-containing periodic mesoporous organosilicas and their catalytic activity in the oxidation of cyclohexane, Microporous and Mesoporous Materials, 99, 334–344, 2007. |
| [32] | J. K. Lee, S. Y. Kim, S. U. Kim, J. H. Kim,Synthesis of cationic polysaccharide derivatives and their hypocholesterolaemic capacity, Biotechnol. Appl. Biochem., 35, 181–189, 2002. |
| [33] | L. Giraldoa, Y. Ladinob, J. C M. Piraján, M. P. Rodríguezb Synthesis and characterization of activated carbon fibers from Kevlar, Ecl. Quím., São Paulo, 32[4], 55-62, 2007. |
| [34] | J. A. Bae, K. Song, J. Jeon, Y. S. Ko, Y. Park, J. Yim, Effect of pore structure of amine-functionalized mesoporous silica-supported rhodium catalysts on 1-octene hydroformylation, Microporous and Mesoporous Materials, 123, 289–297, 2009. |
| [35] | M. Llusar, G. Monrós, C. Roux, J. L. Pozzo, C. Sanchez, One-pot synthesis of phenyl- and amine-functionalized silica fibers through the use of anthracenic and phenazinic organogelators, J. Mater. Chem., 13, 2505–2514, 2003. |
| [36] | Y. Q. Wang, C. M. Yang, B. Zibrowius, B. Spliethoff, M. Linde, F. Schüth, Directing the Formation of Vinyl-Functionalized Silica to the Hexagonal SBA-15 or Large-Pore Ia3d Structure, Chem. Mater., 15, 5029-5035, 2003. |
| [37] | N. Hao, Z. Wu, P. A. Webley, D. Zhao, Synthesis of ordered mesostructured polymer–organosilica composites by the triconstituent co-assembly method, Materials Letters, 65, 624–627, 2011. |
| [38] | X. Wang, Y. Tseng, J. C. Chan, S. Cheng, Direct synthesis of highly ordered large-pore functionalized mesoporous SBA-15 silica with methylaminopropyl groups and its catalytic reactivity in flavanone synthesis, Microporous and Mesoporous Materials, 85, 241–251, 2005. |
| [39] | X. Wang, K. S. K. Lin, J. C. C. Chan, S. Cheng, Direct Synthesis and Catalytic Applications of Ordered Large Pore Aminopropyl-Functionalized SBA-15 Mesoporous Materials, J. Phys. Chem. B, 109, 1763-1769, 2005. |
| [40] | S. Zhu, Z. Zhou, D. Zhang, H. Wang, Synthesis of mesoporous amorphous MnO2 from SBA-15 via surface modification and ultrasonic waves, Microporous and Mesoporous Materials, 95, 257–264, 2006. |
| [41] | N. Liu, R. A. Assink, B. Smarsly, C. J. Brinker, Synthesis and characterization of highly ordered functional mesoporous silica thin films with positively chargeable –NH2 groups, Chem. Commun., 10, 1146, 2003. |
| [42] | V. Antochshuk, M. Jaroniec, 1-Allyl-3-propylthiourea modified mesoporous silica for mercury removal, Chem. Commun., 3, 258, 2002. |
| [43] | T. Yokoi, H. Yoshitake, T. Tatsumi, Synthesis of Anionic-Surfactant-Templated Mesoporous Silica Using Organoalkoxysilane-Containing Amino Groups, Chem. Mater., 15, 4536, 2003. |
| [44] | S. Huh, J.W. Wiench, J. C. Yoo, M. Pruski, V.S.Y. Lin, Organic Functionalization and Morphology Control of Mesoporous Silicas via a Co-Synthesis Tsondensation Method, Chem. Mater., 15, 4247, 2003. |
| [45] | D. A. Loy, G. M. Jamison, B. M. Baugher, E. M. Russick, R. A. Assink, S. Prabaker, K. J. Shea, Alkylene-bridged polysilsesquioxane aerogels: highly porous hybrid organic-inorganic materials, J. Non-Cryst. Solids, 186, 44–53, 1995. |
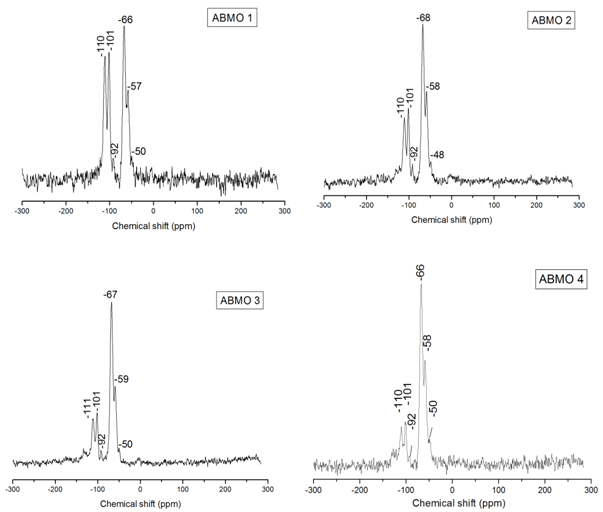
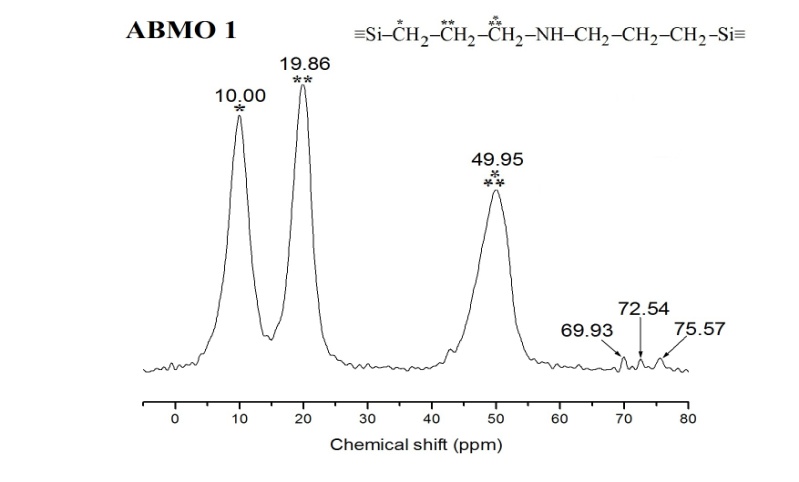
| [46] | T. Asefa, M. Kruk, N. Coombs, H. Grondey, M. J. Maclachlan, M. Jaroniec, G. A. Ozin, Novel route to periodic mesoporous aminosilicas, PMAs: ammonolysis of periodic mesoporous organosilicas, J. Am. Chem. Soc., 125, 11662, 2003. |
| [47] | M. Park, S. S. Park, M. Selvaraj, D. Zhao, C. Ha, Hydrophobic mesoporous materials for immobilization of enzymes, Microporous and Mesoporous Materials, 124, 76–83, 2009. |
| [48] | A. S. M. Chong, X. S. Zhao, Functionalization of SBA-15 with APTES and Characterization of Functionalized Materials, J. Phys. Chem. B, 107, 12650-12657, 2003. |
| [49] | X. Wang, Y. Tseng, J. C.C. Chan, S. Cheng, Direct synthesis of highly ordered large-pore functionalized mesoporous SBA-15 silica with methylaminopropyl groups and its catalytic reactivity in flavanone synthesis, Microporous and Mesoporous Materials, 85, 241–251, 2005. |
| [50] | L. D’Souza, P Devi, D. Shridhar, C. G. Naik, Use of Fourier Transform Infrared (FTIR) Spectroscopy to Study Cadmium-Induced Changes in Padina Tetrastromatica (Hauck), Analytical Chemistry Insights, 3, 135–143, 2008. |
| [51] | W. N. Sivak, I. F. Pollack, S. Petoud, W. C. Zamboni, J. Zhang, Eric J. Beckman, LDI–glycerol polyurethane implants exhibit controlled release of DB-67 and anti-tumor activity in vitro against malignant gliomas, Acta Biomaterialia, 4, 852–862, 2008. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML