-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Modern Botany
p-ISSN: 2166-5206 e-ISSN: 2166-5214
2013; 3(2A): 15-25
doi:10.5923/s.ijmb.201310.03
The Effect of Resource Stress on Goldenrod’s Tolerance of Folivory Depends More on the Identity of the Stress than on the Severity of the Stress
Peter J. March 1, 2, Michael J. Wise 1, 3, Warren G. Abrahamson 1
1Department of Biology, Bucknell University, Lewisburg, PA 17837, USA
2Arthur A. Dugoni School of Dentistry, University of the Pacific, San Francisco, CA 94115, USA
3Department of Biology, Roanoke College, Salem, VA 24153, USA
Correspondence to: Michael J. Wise , Department of Biology, Bucknell University, Lewisburg, PA 17837, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
It is widely accepted that the levels of resources in a plant’s environment can influence the plant’s ability to compensate for (i.e., tolerate) damage by herbivores. However, predicting the direction of the influence has proven difficult. Here, we report on a greenhouse study in which individuals of Solidago altissima were exposed to factorial combinations of light and fertilization levels to investigate how different types of stresses affect plants’ ability to tolerate leaf damage by larvae of the beetle Trirhabda virgata. Shade stress reduced the plants’ tolerance of herbivory, while nutrient stress had no effect on tolerance. These results did not completely fit the predictions of any of the three predominant models for the effects of resource levels on herbivory tolerance, but they were best explained by the Limiting Resource Model (LRM). We discuss why the results of this study, as well as some other recent studies, may not exactly fit the predictions of the LRM. We highlight examples in which anomalous results have led to novel insights into factors that affect plants’ ability to compensate for herbivore damage.
Keywords: Compensatory Continuum Hypothesis, Growth Rate Model, Herbivory Tolerance, Limiting Resource Model, Nutrient Stress, Shade Stress, Solidago altissima, Trirhabda virgata
Cite this paper: Peter J. March , Michael J. Wise , Warren G. Abrahamson , The Effect of Resource Stress on Goldenrod’s Tolerance of Folivory Depends More on the Identity of the Stress than on the Severity of the Stress, International Journal of Modern Botany, Vol. 3 No. 2A, 2013, pp. 15-25. doi: 10.5923/s.ijmb.201310.03.
Article Outline
1. Introduction
- Although herbivory is one of the most common and important ecological interactions on the planet[1], ecologists have long been at odds regarding how strongly herbivores affect the fitness of their host plants[1-7]. In particular, the fitness impact of herbivore damage can range from strongly negative, to neutral, to occasionally beneficial—even in the same plant species exposed to the same type of damage [8-11]. This paradox was partially resolved with the general acceptance that the ability of a plant to tolerate herbivore damage depends on the environmental conditions (e.g., resource levels) under which the plant is living[12,13]. However, the questions of how and why resource levels affect tolerance of herbivory remain topics of vigorousdebate [14-16].The predominant school of thought among evolutionary ecologists has been that plants in relatively high-resource or low-competition environments will have more resources available for compensatory growth and reproduction in response to herbivore damage. This rather intuitive expectation of higher resource levels leading to greater tolerance of herbivory has been formalized as the Continuum of Response Model or, more commonly, the Compensatory Continuum Hypothesis (CCH). The CCH is generally credited to Maschinski & Whitham[12]and Whitham et al. [13], although similar rationale was central to the influential hypotheses on plant defense proposed by Bryant, Chapin &Klein[17] and Coley, Bryant & Chapin[18]. A parallel school of thought, developed in the context of grazing optimization, makes the opposite prediction of the CCH:Tolerance of herbivory should be greater in more stressful, low-resource environments[19-22]. The rationale for this hypothesis, commonly called the Growth Rate Model (GRM), is that plants in low-resource conditions are growing well below their maximum potential and thus have more room to increase their growth rates, catch up with the undamaged plants, and thus compensate for herbivore damage.As empirical studies of the effect of resource levels on tolerance of herbivory have accumulated, it appears that resource stress is just as likely to increase a plant’s ability to tolerate herbivory as it is to decrease its tolerance[14,15,23]. Therefore, neither the CCH nor the GRM alone is sufficient to explain the diversity of empirical results.In an attempt to reconcile the paradoxically contrasting effects of resource stress on plant tolerance, Wise & Abrahamson[24] proposed a model with more flexibility than the CCH or GRM. The central rationale of their model was that plant performance in any environment, regardless of herbivory, is likely to be restricted by the single resource that is in most limited supply relative to the plant’s needs; thus, the model is called the Limiting Resource Model. This rationale is not new,of course, as it is based on the classic Sprengel-Liebig Law of the Minimum[25,26]. However, the LRM was the first comprehensive attempt to apply this rationale to explain how resource stress affects plant tolerance of herbivory.The LRM includes several simple considerations that the CCH and GRM do not explicitly take into account when predicting herbivore tolerance in high- vs. low-resource conditions[15,24,27]. The LRM considers not just the relative level of the resource, but the identity of the resource and whether this resource is the one most limiting plant performance. The LRM also considers the type of herbivory and how the herbivore’s damage affects resource acquisition by the plant. Finally, the LRM considers whether the damage is likely to exacerbate an existing resource limitation, create a new resource limitation, or have no effect on the limiting resource.Consider the application of the LRM to predict a plant’s relative tolerance of leaf herbivory (folivory) in low- vs. high-resource environments. For example, in a shaded environment, plant fitness is more likely to be carbon-limited than in a full-sun environment, where fitness is likely to be limited by another resource (e.g., nitrogen). Folivory is likely to mainly affect plants’ ability to photoassimilate carbon. Thus, a given amount of folivory is likely to exacerbate the acquisition of the limiting resource to a greater extent in the shaded environment than it would in a full-sun environment. Therefore, the LRM would predict greater tolerance of folivory in the full-sun environment.Consider now the same amount of folivory in a low-nitrogen vs. a high-nitrogen environment. Nitrogen is more likely to be limiting in the low-nitrogen environment than in the high-nitrogen environment, and thus a different resource—perhaps carbon—is more likely to be the resource limiting plant fitness in the high-nitrogen environment.If carbon is indeed more limiting in the high-nitrogen environment, then folivory is likely to have a more negative impact in the high-nitrogen environment, where it would be exacerbating the existing resource limitation. Therefore, the LRM would predict greater tolerance of folivory in the low-nitrogen environment. In contrast, the CCH would predict greater tolerance in both the full-sun and high-nitrogen environments, while the GRM would predict greater tolerance in both the shaded and low-nitrogen environments.In a recent review, Wise & Abrahamson[15]applied the pathways of the LRM in an attempt to explain the results of 41 studies of the effects of resource (light, nutrients, or water) level on tolerance of folivory. Consistent with the LRM predictions outlined above, when the limiting resource in the environment was light, tolerance of folivory was generally greater in the high-light environment across the studies. When the limiting resource was nutrients (or water), tolerance of folivory was generally greater in the low-nutrient (or water) environment than in the nominally high-resource environment.While this review[15], and a similar review on tolerance of apical-meristem damage[27], demonstrated that the considerations of the LRM can be quite useful in explaining a myriad of past results, such reviewsare somewhat limited in terms of assessing the LRM’s predictive power. Specifically, the interpretations of the studies relative to the LRM were necessarily retrospective, as none of the studies were designed specifically to test the LRM and thus did not always provide all the details that were desirable. Furthermore, the studies used a wide variety of plant species, herbivore species, levels of stress, and experimental protocols. While having such a comprehensive group of studies allows for robust general conclusions, it makes direct comparisons among studies difficult. For example, rarely did a single study look at the effects of different types of resource stress on tolerance of folivory of a single plant species under otherwise identical conditions[but see 28].The main goal of the current study was to test the predictions of the three tolerance models for plants exposed to folivory under stress from shortages of two different types of resources. We exposed individuals of tall goldenrod, Solidago altissima, to three levels of folivory by the chrysomelid beetle Trirhabda virgata in a greenhouse experiment in which light level and inorganic-nutrient levels were varied in a factorial fashion. We tested the tolerance predictions of the three models: 1) CCH—Plants will have higher tolerance of folivory in both the high-light and high-nutrient treatments than in the low-light and low-nutrient treatments; 2) GRM—Plants will have higher tolerance in both the low-light and low-nutrient treatments than in the high-light and high-nutrient treatments; and 3) LRM—The effect of resource stress on tolerance of folivory will differ between light and nutrient experiments. Specifically, tolerance will be greater in the high-light than the low-light treatment, where carbon is likely to be more limiting; and tolerance will be greater in the low-nutrient treatment than the high-nutrient treatment, where carbon is likely to be more limiting.
2. Materials and Methods
2.1. Study System
- Solidago altissima L. (Asteraceae), or tall goldenrod, is a perennial herb abundant in disturbed sites, old-fields, and roadsides throughout the eastern United States and southern Canada[29]. In central Pennsylvania, the site of this study, ramets (individual shoots) of S. altissima emerge from rhizomes in late April and early May. This species is noted for its prodigious bloom of small yellow flowers in early autumn. The flower heads (or capitula) contain female ray and bisexual (perfect) disk flowers, or florets. Both types of florets produce single-seeded, wind-dispersed achenes, which mature and begin to disperse near the beginning of November. Solidago altissima relies on a variety of insect visitors for pollination, but the flowers will produce sterile-seeded achenes even without pollination[30].The insect-herbivore community of S. altissima has received considerable study[31-34]. Beetles of the genus Trirhabda, especially T. virgata, are among the most damaging folivores of S. altissima, occasionally nearly completely defoliating entire fields of goldenrod[35-41]. The overwintering eggs of T. virgata hatch from soil or dead vegetation in spring, and larvae crawl onto young goldenrod ramets to begin feeding. Their feeding creates characteristic, roughly semi-circular holes on the edges of leaves[42,43]. Larvae feed on leaves for approximately 3-4 weeks then crawl underground to pupate[23]. Adult beetles emerge 2-3 weeks later and continue to feed on goldenrod leaves and lay eggs in late summer and early autumn[43-45].
2.2. Experimental Design
- The plant material used for this experiment came from 26 genets whose rhizomes were originally excavated from a 3-ha field in Union County, PA (N40º 57.8’, W76º 57.5’) in early April of 2003. The rhizomes were cut into segments and planted in flats in commercial growing medium (ProMix BXTM, Premier Horticulture, Dorval, Quebec, Canada). In order to produce many new rhizomes, ramets that grew from the flats were transplanted to 27-cm plastic standard pots in a greenhouse where they grew until senescence in late autumn. The new rhizomes were removed from the pots and refrigerated over winter. In the spring of 2004, segments of rhizomes were cut from each of the 26 genets and were again grown in Pro-Mix BX™ in 27-cm plastic standard pots on wooden pallets in a semi-protected, unshaded area outside of the greenhouse until senescence in late 2004. The new rhizomes were then refrigerated and used for planting in March of 2005. Ten of the 26 genets were randomly chosen for the current experiment.The rhizomes were cut into equal-volume segments of 2 cm3, which were measured using water displacement in a 100-ml graduated cylinder. The rhizome segments were planted in flats with Pro-Mix BX™. In early-May, at least 50 healthy ramets from each of the 10 genets were transplanted into 17.5-cm plastic azalea pots filled with Pro-Mix BX™. If a transplanted rhizome initiated more than one ramet, all but the largest were removed as soon as possible after emergence.For each of the 10S. altissima genets, 48 ramets were selected for the experiment on 2 June 2005. The experiment was conducted on eight greenhouse benches, which were grouped into four blocks of two benches. One of two benches per block was randomly chosen to receive the low-light and one the high-light treatment. To accomplish the low-light treatment, the bench was shaded using a PVC frame covered with shade cloth that blocked 50% of ambient light. The high-light treatment was uncovered, such that the ramets received ambient greenhouse light.Within each bench, 60 ramets (six for each of the 10 genets) were randomly assigned factorial combinations of two nutrient and three herbivory treatments, and the positions of the ramets were randomly assigned in six rows of 10 ramets.The ramets that were assigned to the high-nutrient treatment were fertilized weekly (starting on 23 June) with 2.5-strength Hoagland’s solution[46]. This solution supplied the ramets with an estimated 1.3 times the amount of nutrients that typical field ramets receive[47]. The ramets in the low-nutrient treatment did not receive any fertilizer during the experiment. All ramets across all treatments were watered ad libitum.The herbivory treatment consisted of three levels: a control level with no herbivory, a low-herbivory treatment of 33% leaf-area removal, and a high-herbivory treatment of 67% leaf-area removal. Because manually clipping leaves may not induce herbivore defenses the same way that beetle chewing does, a mix of real and simulated herbivory was used to mimic natural damage while precisely controlling the amounts of damage[48,49].To initiate the folivory treatments, three field-collected, first-instar T. virgata larvae were placed on each of the 33% and 67% damage-treatment ramets in early June. After 2-3 weeks of feeding, the larvae were removed during their last instar to prevent pupation and emergence of adults in the greenhouse. The damage was then standardized for each herbivory-treatment level with scissors. For the 33% damage level, the right half of two out of every three leaves was clipped, starting at the base of the stem and ending with the last fully expanded leaf near the apex. For the 67% damage level, the right half of two out of every three leaves was clipped and the third leaf had both halves removed, leaving only the midribs, as T. virgata tend not to consume the midribs[45]. To simulate the timing and damage of adult beetles, a second round of manual defoliation was performed between 14-19 July. The clipping patterns were the same as for the first round of defoliation, except that the clipping started on the first fully expanded leaf since the first damage treatment and ended with the last fully expanded leaf near the shoot apex.The 120 ramets from one of the four blocks were sacrificed for chemical analyses prior to the end of the experiment. These analyses are planned for a future contribution and will not be mentioned further in this paper. The experiment thus included three blocks and a total 360 ramets for which fitness measurements and tolerance analyses were performed.
2.3. Data Collection
- For this study, we used an estimate of seed production per ramet as the fitness proxy for analyzing the ramets’ tolerances. Because S. altissima is a perennial herb, seed production represents just one episode of sexual reproduction, but we assume that this episode is representative of episodes that the plants would experience in other years. Because S. altissima is self-incompatible, seed production is a measure of maternal fitness. We do not estimate paternal fitness (e.g., siring success through pollen) in this study. Finally, because the flowers were not pollinated in this experiment, the seeds formed were not viable. We assume that results would be similar if the seeds were viable, though they might be expected to contain a different resource profile than sterile seeds.Seed production for a ramet was estimated in a three-step process. First, after the ramet set seed, the infructescence branches were removed one at a time and the number of capitula (seed heads) were counted by hand. Second, 10 capitula were collected from different areas of the infructescence, and the capitula were dissected under a microscope to count the number of achenes and obtain the mean achene number per capitulum. (Each achene contains one seed.) Third, the mean achene number per capitulum was multiplied by the number of capitula for the ramet to calculate the total seed production per ramet.
2.4. Statistical Analyses
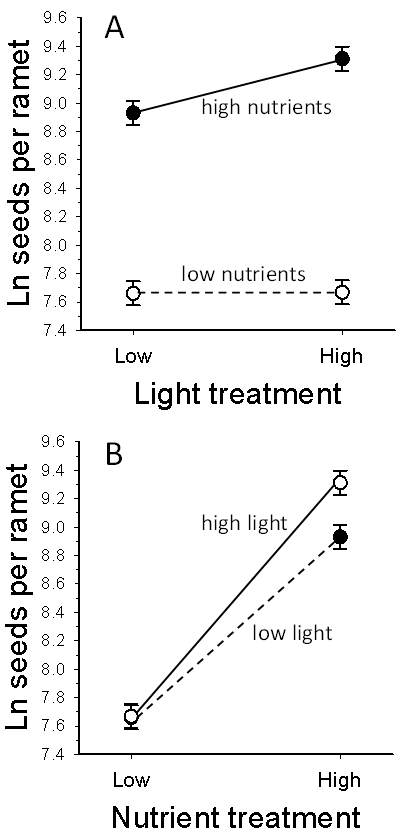
- The seed-production results were analyzed using an analysis of covariance (ANCOVA) with a split-plot design. Seed production was natural-log transformed to meet the distributional assumptions of ANCOVA. Moreover, log-transformation has the effect of putting fitness on a multiplicative (or proportional) scale to match the proportional metric for herbivore damage (i.e., % of leaf area removed)[50]. Because several ramets produced zero seeds, we added 100 to the seed number for each ramet prior to taking the logarithms, which had the effect of reducing the extremity of the zero-seed outliers.In the split-plot design, the light treatment was the main plot. Block was considered the experimental unit for the main plots. Thus, the ANCOVA included light as a fixed-effects factor and block-within-light as a random-effects factor. The split plots (or subplots) included the three other main factors (nutrients, herbivory, and genet), which were factorially crossed within the light-treatment main plot. Genet was treated as a random factor, nutrients were a fixed factor, and herbivory was treated as a covariate, taking on the values of 0, 0.33, or 0.67. All two-way interactions between factors were included in the ANCOVA. The interactions between light and herbivory and between nutrients and herbivory revealed whether tolerance of herbivory was affected by resource level. The two-way interactions with genet, which were also treated as random factors, indicated whether there was genetic variation for tolerance of herbivory, shade stress, or nutrient stress. The interaction between light and nutrients provided insight intowhether the resource treatments were effective in changing resource limitations. That is, they indicated whether the addition of nutrients made the plants relatively more light-limited, and vice versa. Three-way and four-way interactions were also considered but were dropped from final model because they offered no substantive interpretation, and none were close to statistical significance.The ANCOVA was performed with JMP-IN 4.0.4 (SAS Institute, Cary, North Carolina, USA) using both the traditional EMS (expected mean square) technique and the restricted maximum likelihood (REML) technique for model estimation. For each factor, the EMS technique calculates denominator mean squares and degrees of freedom using the linear combination of the other mean squares that has the same expectation as the mean square of the effect under the null hypothesis. JMP-IN indicated that there were problems in the iterations used in the REML technique that may have led some results to be invalid. Therefore, we only present results from the EMS technique. Notably, the inferences (P-values) were nearly identical for all of the factors of interest for both estimation techniques.
3. Results
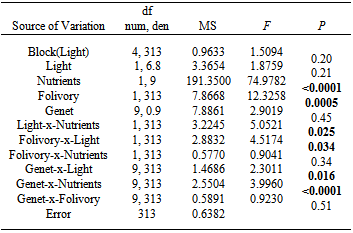
- Leaf damage by T. virgata negatively affected the fitness of S. altissima as measured by seed production (P = 0.0005, Table 1). Removal of one-third of the leaf area depressed seed production by 12%, while removal of two-thirds decreased seed production by 27%.
|
4. Discussion
4.1. Goldenrod Experiment
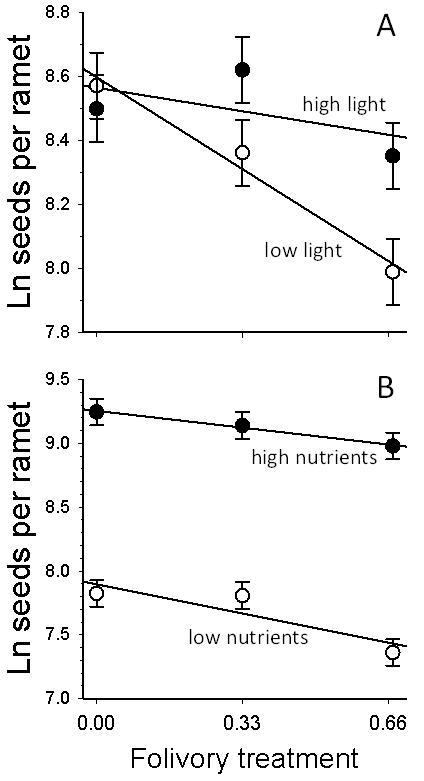
- In this study, the defoliation treatment (Trirhabda feeding plus manual clipping) significantly reduced fitness of Solidago altissima. Nevertheless, the plants showed an impressive degree of tolerance of the defoliation: The loss of two-thirds of a ramet’s leaf area led to only a 27% reduction in mean seed production. As expected, the plants’ ability to tolerate damage was affected by the resource conditions of the environment in which they were growing. However, the effects of resource stress on tolerance of folivory were not simple. They depended not just on the presence or absence of stress, but on the identity of the resource that was in limited supply. While this dependence of herbivory tolerance on the identity of the resource is not predicted by either the Growth Rate Model (GRM) or the Compensatory Continuum Hypothesis (CCH), it is a central prediction of the Limiting Resource Model (LRM). The results, however, were not completely consistent with the LRM’s prediction either.Below, we argue that more insight is generally gained when results do not quite fit the predictions of simple models.At full (ambient greenhouse) light, S. altissima was able to tolerate folivory without an effect on seed production. At reduced light (50% of ambient), plants were significantly less tolerant of folivory. This result is the opposite of what the GRM predicts. The rationale of the GRM is that, because all the plants in the shade would be growing suboptimally (well below their potential), shaded plants that are damaged would not have as far to catch up to match the seed production of the undamaged shaded counterparts. By contrast, in full light, plants would be growing more quickly, so damaged plants would have farther to go to catch up with the undamaged plants. Thus, the GRM would predict that tolerance of folivory would be greater in the shade-stressed environment. In contrast, the shade results were consistent with the prediction of the CCH. Simply put, the rationale of the CCH is that the full-light plants were more robust in general than the shade-stressed plants. Thus, they are in a better position to allocate resources to tolerate any stress they might face, including defoliation.The LRM makes the same prediction as the CCH for the shade treatment, but using a more nuanced rationale. Specifically, the shaded plants are likely to be more carbon-limited than the high-light plants. Thus, defoliation, which should mainly affect carbon assimilation, is likely to have a more severe effect on seed production in the shaded plants than in the high-light plants.Although nutrient stress had a much more severe impact on seed production than shade stress in this study, there was no evidence that nutrient stress influenced the plants’ ability to tolerate defoliation. This lack of effect is not predicted by any of the three simple tolerance models. The GRM would predict that because the unfertilized plants were growing well below their potential, those that suffered folivory would not have to do much to catch up with their undamaged, unfertilized counterparts. Thus, the unfertilized plants would be predicted to be more tolerant of damage than the fertilized plants. In contrast, the CCH would predict that the fertilized plants, which were much larger and more robust, would be more capable of compensatory growth than the smaller, unfertilized plants. Thus the CCH would predict higher tolerance in the fertilized plants.The LRM considers that the fertilized plants are less likely to be nutrient limited than the unfertilized plants; thus, the fertilized plants are more likely to be carbon limited than the unfertilized plants. Again, since defoliation is expected to mainly affect carbon assimilation, defoliation is predicted to have a more severe effect on the seed production of the fertilized than the unfertilized plants. The equal tolerance of the fertilized and unfertilized plants thus contradicts the LRM’s prediction, as well as those of the GRM and CCH, for the nutrient part of this experiment.Before attempting to interpret the nutrient results of the current study, it is instructive to examine the results of a prior studyby Meyer and Root[23] on the effects of nutrient stress on S. altissima’s tolerance of defoliation by Trirhabda beetles.In that study, herbivore damage only reduced seed production when the plants were growing under high soil fertility. The plants in the low fertilizer treatment were completely tolerant of defoliation by Trirhabda. This result was surprising because it was one of the earliest, clearest contradictions of the intuitively appealing prediction of the CCH. Meyer and Root[23] interpreted their results by considering the relative strength of carbon and nutrient limitations in the two fertilizer levels. Specifically, they reasoned that reproduction of the highly fertilized plants was more likely to have been carbon limited, while reproduction in the low-fertilizer group was more likely to have been nutrient limited. Herbivory thus was more likely to have a stronger impact in the carbon-limited, high-fertilizer group, and less of an impact in the nutrient-stressed group. This is the same rationale that inspired the construction of the Limiting Resource Model as a formal framework for explaining and predicting the effects of resource stresses on tolerance of herbivory[24]. This result of higher tolerance of leaf damage in nutrient-stressed plants has been found repeatedly in a wide variety of species, including both monocots and dicots[15].Given Meyer and Root’s[23] results, it is perhaps ironic that our current study—the first designed specifically as a comprehensive test of the LRM versus the CCH and GRM—failed to support the prediction of the LRM regarding the effect of nutrient stress on tolerance of folivory. For an explanation, we will examine the two main premises that lead to the LRM’s prediction: 1) that the unfertilized plants were carbon stressed, and 2) that the resource primarily affected by folivory was carbon. Our study only employed two levels of nutrient treatment: no fertilizer and a regimen of fertilization meant to mimic nutrient levels slightly higher than typical field levels. With their greatly stunted growth and reproduction, the unfertilized plants in the study were clearly nutrient stressed. However, it is possible that reproduction of the fertilized plants wasalso nutrientlimited.Had we used higher levels of fertilizer, we may have increased seed production even further. Without multiple fertilizer levels, we cannot know for sure if our fertilizer treatment flipped seed production from being nutrient limited to carbon limited.There are at least two main reasons why the second premise may not hold. First,leaves contain compounds constructed of (and host metabolic processes involving) elements other than carbon. Second, plants tend to be excellent at integrating their resource use—altering their growth patterns in a variety of ways to maintain a balance in resource acquisition[51-54]. For instance, a plant that is shade stressed may grow taller or produce larger leaves than plants with ample light[55,56], while a plant that is nutrient or water stressed may increase its root: shoot ratio[57-60]. In addition, a plant whose leaves are damaged by herbivores will tend to reduce allocation to root growth in favor of shoot regrowth, thus ameliorating the resource imbalance caused by the folivore[61-63].Given this plasticity in growth, predicting the resource most affected by herbivory can be trickier than first imagined. The damage must be severe enough to overcome the plant’s natural ability to balance its resource acquisition with its needs. Nevertheless, any model that attempts to explain a complex ecological interaction (like the effect of resource stress on tolerance of herbivory) must face a tradeoff between explanatory power and simplifying assumptions. Even so, literature reviews have shown that the extra considerations of resource identification in the LRM more often than not have enabled the LRM to explain empirical results that do not match the predictions of the CCH or of the GRM[15,27].
4.2. A Retrospective and Prospective Look at the Tolerance Models
- In this special issue on the evolutionary ecology of plant defense, we want take the opportunity to give more credit to the CCH and GRM than is generally possible in empirical papers, where economy of space dictates the need for simplification. In particular, the nuances of the backgrounds and intentions of the CCH and GRM have rarely been acknowledged in recent papers on the tolerance of herbivory. Evolutionary ecologists will certainly continue to use the simplified versions of the CCH and GRM as heuristic foils because they so conveniently make the exact opposite predictions. Nevertheless, these naïve predictions belie the actual success of the models, which is best evidenced by the enormous amount of empirical research and additional questions that they have generated.The origin of the CCH as used by evolutionary ecologists can be traced back mainly to two influential papers by Maschinski, Whitham, and colleagues[12,13]. These authors discussed several factors that could influence plants’ ability to compensate for damage, including timing of damage, size of the plant, type of tissue damaged, and intensity of herbivory, in addition to environmental conditions. What was later named the CCH—or the Continuum of Response Model[64,65]—was a simplification of one of the predictions made in the paper: tolerance of herbivory should be greater in high-resource environments than low-resource environments.The GRM also has a richer and more complex history than the caricature often used by evolutionary ecologists. The GRM arose from literature on grazing and, in particular, models that attempted to explain conditions under which grazing may lead to increased production (i.e., grazing optimization)[20,22,66]. One paper in particular by Hilbert et al.[19] exemplified the prediction of greater impact of grazing in resource-rich environments that would become known as theGrowth Rate Model[65]. Importantly, while evolutionary ecologists are more interested in fitness effects in natural systems, the originators of the GRM were interested in productivity effects in managed systems. When we introduced the Limiting Resource Model[24], we were fortunate to be able to name it ourselves and to specify its intended applications. The details of the original formulation of the LRM (with its multiple pathways) was an attempt to explain in a comprehensive fashion the otherwise seemingly complicated mix of results of prior empirical studies on the effects of resource stress on tolerance of herbivory. While the results of a few of the studies in our reviews could not be explained by employing the LRM, we demonstrated that by asking a few simple questions regarding limiting resources, one could make sense out of the results of numerous studies that could not be adequately explained by the CCH or GRM[15,27]. In doing so, we demonstrated that we do not have to abandon the idea that resource levels can have a predictable effect on tolerance of herbivory. In addition, we showed that it was the identity of the resources involved, rather than the type of plant (e.g., monocot vs. dicot), that was the main determinant of the effect on tolerance.The reactions to the LRM have followed a typical pattern for new hypotheses. Since its introduction, numerous authors have used the LRM to explain results of their empirical studies on the effects of resource stress on herbivory tolerance[67-75]. Some have expanded the reach of the LRM by applying it to a diversity of new basic and applied contexts, including plant-community-level responses[76]; effects of succession[77]; silviculture practices and reforestation projects[78,79]; biological-control strategies for invasive plants[80-83]; responses to atmospheric increases in carbon dioxide[84-86]; and effects of eutrophication in aquatic environments[59,69]. At the same time, quite a few other authors have highlighted empirical results that were not predicted by the LRM[e.g., 85-89]. Despite the relatively more complex formulation of the LRM than the simplified formulations of the CCH or GRM, only rarely has the LRM been misrepresented or misapplied in the literature[e.g., 90].
5. Conclusions
- With the growing appreciation that there is nothing fundamentally new or controversial about it,we expect that the LRM will approach the level of orthodoxy in the field of herbivory tolerance[16]. At its core, the LRM simply applies the rationale of the Sprengel-Liebig Law of the Minimum, which dates back to the mid-1800s[26], to plant tolerance of herbivory.As with any useful model, we maintain that the cases in which the LRM’s predictions fail are the ones that can shed the most insight into tolerance by identifying in which areas gathering more data would be most illuminating. For instance, the results may suggest that one needs to look more closely into the range of effects that herbivores have on their host plants, or the range of strategies plants use to alter their acquisition of different resources. One of the most productive strategies for using the LRM in this way has been to investigate why resource stresses may affect herbivory tolerance in different ways in different plant species[60,67, 70,91,92].The application of the LRM—particularly in cases in which the results did not follow the simple predictions—has led several authors to interesting new insights into factors that affect tolerance of damage. These factors include the influence of symbionts, such as mycorrhizal fungi[93], nitrogen-fixing bacteria[94], and endophytic fungi[95,96]; the effects of other biotic interactions, such as pollination and nectar robbing[97] and nurse plant-protégé interactions [98]; the effects of physical stresses, such as thesoil depth from which seeds germinate[99], burial of plant parts by sand[73], and suppression of jasmonic acid signaling by elevated carbon dioxide levels[84]; and the influence of transgenerational, maternal effects[100]. As with the CCH and GRM before it, we hope that the LRM will continue to lead to further research and new hypotheses that lead to a more complete and sophisticated understanding of factors that influence plants’ ability to tolerate herbivory.
ACKNOWLEDGEMENTS
- This study was carried out in partial fulfilment of the requirements of P. March’s MS degree in Biology at Bucknell University. We thank C.P. Blair, N. Dorchin, M.E. McTammany, and M.D. Spiro for input throughout the project, D.E. Carr for advice on statistical analyses, and S.E. Wise for additional technical and editorial support during preparation of the manuscript. We thank Rolf and Anick Helbig for permission to collect goldenrod rhizomes on their property and the New York Department of Environmental Conservation for permission to collect Trirhabda virgata at the Upper and Lower Lakes Wildlife Management Area. We also thank L.E. Coffey, A. Latimer, and B. LaFlammefor assistance counting and dissecting goldenrod capitula.Our work was supported financially by the David Burpee Endowment of Bucknell University and National Science Foundation grants (DEB-0515483) to WGA and MJW and (DEB-0343633) to WGA and J.T. Irwin. Any opinions, findings, and conclusions expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML