-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Energy and Power
p-ISSN: 2163-159X e-ISSN: 2163-1603
2014; 4(1A): 50-56
doi:10.5923/s.ep.201401.04
Modeling Methanol Steam Reforming for Internal Combustion Engine
Leonid Tartakovsky, Vladimir Baibikov, Mark Veinblat
Faculty of Mechanical Engineering, Technion – Israel Institute of Technology, Haifa 32000, Israel
Correspondence to: Leonid Tartakovsky, Faculty of Mechanical Engineering, Technion – Israel Institute of Technology, Haifa 32000, Israel.
| Email: | 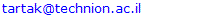 |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Methanol steam reforming is studied using the thermodynamic equilibrium assumption and the Gibbs free energy minimization method. Different reaction paths of methanol steam reforming are simulated and analysed. An empiric correlation derived on the base of previous experimental studies is suggested to bring the simulation results closer to the actual findings. Composition of the methanol steam reforming products is analysed for different temperatures and water to methanol ratios. Energy analysis is carried out to determine the optimal conditions of the methanol steam reforming for powering an internal combustion engine.
Keywords: Thermo-chemical recuperation, Methanol, Steam reforming, Energy analysis, Hybrid propulsion system
Cite this paper: Leonid Tartakovsky, Vladimir Baibikov, Mark Veinblat, Modeling Methanol Steam Reforming for Internal Combustion Engine, Energy and Power, Vol. 4 No. 1A, 2014, pp. 50-56. doi: 10.5923/s.ep.201401.04.
Article Outline
1. Introduction
- Energy efficiency improvement is one of society's most important instruments for mitigating climate change. Transportation is responsible for a large part of the energy consumption worldwide. According to International Energy Agency (IEA) data, about 26% of all energy-related CO2 emissions were caused by transportation[1]. It is likely to account for a higher share in the future, unless special measures are taken. To limit the long-term global heating down to acceptable levels, United Nations Intergovernmental Panel on Climate Change (IPCC) recommended that annual global greenhouse gas (GHG) emissions must be reduced by 50 - 85% by year 2050 in comparison with the emissions level in 2000[2]. According to the IEA's BLUE map scenario[1], above 80% of the projected GHG emission mitigation may be achieved by improvement of vehicle efficiency, introduction of alternative fuels and electricity decarbonization.Results of recent studies suggest that there is a big potential for improvement of internal combustion engine (ICE) technologies[3-6]. It is well known that about 30% of fuel energy introduced to ICE is wasted with engine exhaust gases[3]. Even its partial utilization could lead to a significant improvement of ICE energy efficiency.One of the ways to recover an engine's waste heat is by using the energy of the exhaust gases to promote endothermic reactions of alcohol steam reforming – ASR[7, 8]. In principle, any renewable fuel may be used, not only alcohol. ICE is fed by the gaseous products of ASR, mainly mixture of hydrogen and carbon monoxide, frequently called syngas. The latter has, as a rule, greater heating value than the primary liquid fuel and may be more efficiently burned in the engine compared with the original fuel. This approach, called thermo-chemical recuperation (TCR), is considered nowadays as one of the possible methods of powertrain efficiency improvement and emissions reduction[7].Many studies published on TCR are focused on gas turbine applications[9-11]. Ivanic et al.[12] studied a partial fuel reforming for ICE. Computational analysis of a spark ignition (SI) engine performance was carried outby Galloni and Minutillo[13] for partial gasoline replacement by a reformate gas. In their work the reformate gas was produced by exothermic partial oxidation of gasoline rather than bio-fuel.Alcohol reforming to a syngas is widely investigated today as a promising hydrogen source for propulsion systems based on fuel cells[14-16]. The thermodynamics of methanol reforming has been extensively discussed in the literature[17-19]. The methanol steam reforming process may be described by three main reactions, namely, methanol decomposition (1), water gas shift -WGS (2) and direct methanol steam reforming (3):
 | (1) |
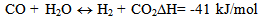 | (2) |
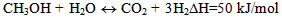 | (3) |
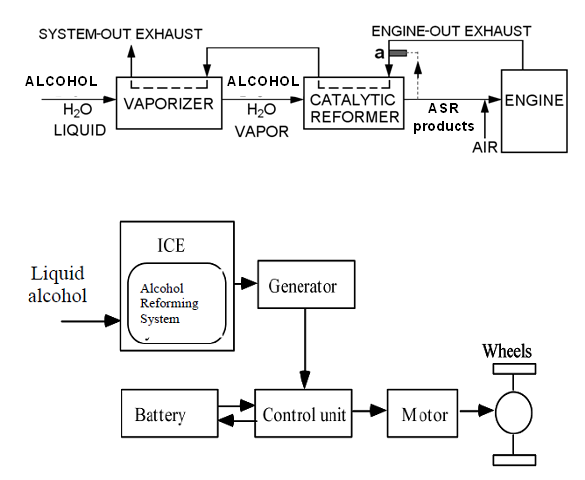 | Figure 1. Hybrid propulsion system with a reformer-ICE |
2. Methodology
- In fuel cell applications dealing with alcohol steam reforming an effort is focused on achievement of maximal possible hydrogen outcome together with prevention of CO formation, which is a poison for the fuel cell catalyst. For these purposes, the overall reaction (3) is preferable, thus implying that the WGS reaction extent is very high. In contrast with the strict requirement of high-purity hydrogen typical for fuel cells application, ICE is much more flexible and can effectively burn different mixtures of hydrogen, carbon monoxide and other gases. This feature greatly reduces the cost of energy obtained from renewable fuels. In this case methanolreforming to CO2 and H2 is undesirable, because CO2 is a diluent gas and does not carry energy. Therefore, for ICE fuelling, realization of the reaction (1) would be preferable with negligible WGS reaction extent. In ICE the exhaust heat is used to promote on-board reforming of methanol into a mixture of hydrogen and carbon monoxide with small amounts of diluents and contaminants (carbon dioxide, unreacted water, etc.). Because this gas has a greater heating value and may be more efficiently burned than the primary fuel, an improvement in engine fuel economy can be expected along with a sensible emissions reduction. CO formed in reaction (1) does not constitute an environmental hazard because it is further oxidized to CO2 during the combustion in the engine. Therefore, for ICE application methanol reforming process has to be optimized for the maximal yield of hydrogen and CO together with prevention of contaminants formation.One important parameter that should be used in reformer optimization is the energy efficiency of the reforming process[24] defined as a ratio of the added enthalpy of combustion (Hout - Hin) to the heat duty, Hd (the sum of the ASR reactions heat, latent heat of methanol vaporization and sensible heat):
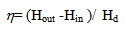 | (4) |
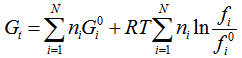 | (5) |
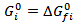 are assumed, where ∆G0fi is the standard Gibbs free energy of formation of species i.The reaction paths including reactions (1) – (3) as well as carbonation, dehydrogenation, olefine and formaldehyde synthesis, methanation and other reactionswere considered. For the analysis simplification the following main assumptions have been made:• Chemical equilibrium in the reactor;• Ideal heat exchanger;• All gases are ideal.The products of the reforming were calculated for a reactor with minimum Gibbs free energy, when all the possible products were taken into account under the following working conditions of the reactor: atmospheric pressure, water-to-methanol molar ratio 0.6-3 and temperature range of 150-950°C.
are assumed, where ∆G0fi is the standard Gibbs free energy of formation of species i.The reaction paths including reactions (1) – (3) as well as carbonation, dehydrogenation, olefine and formaldehyde synthesis, methanation and other reactionswere considered. For the analysis simplification the following main assumptions have been made:• Chemical equilibrium in the reactor;• Ideal heat exchanger;• All gases are ideal.The products of the reforming were calculated for a reactor with minimum Gibbs free energy, when all the possible products were taken into account under the following working conditions of the reactor: atmospheric pressure, water-to-methanol molar ratio 0.6-3 and temperature range of 150-950°C.3. Results and Discussion
3.1. Equilibrium Simulation Results
- The examples of the calculation resultsobtained with the assumption of chemical equilibrium are shown in Figure 2.
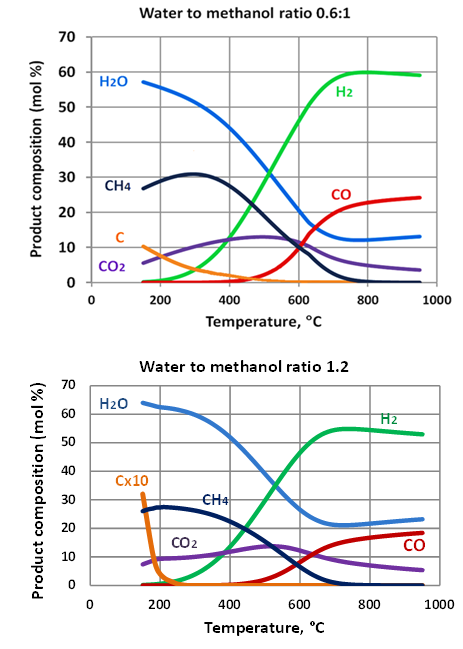 | Figure 2. Methanol reforming products – equilibrium assumption |
3.2. Comparison with Available Experimental Data
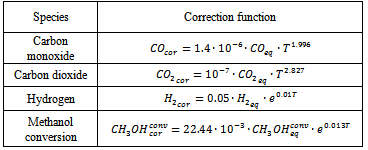
- The available experimental results[28-31] show that the actually measured methanol reforming products are usually composed of: hydrogen, carbon monoxide, water and carbon dioxide (Figure 3). Methane normally does not present between the reforming products. The experimental results also show that the methanol conversion is less than 100% under the temperatures lower than 300 oC (Figure 3). The methanol conversion increases with the temperature rise until full conversion is reached. Full conversion of methanol is usually observed at temperatures of 300-320 oC.
3.3. Empiric Correction of Equilibrium Predictions
- To improve accuracy of the equilibrium predictions and account for the non-equilibrium reforming behaviour, empiric correction of the simulation results was applied. The empiric correction functions were developed using available experimental results for copper-based catalysts[28, 29]. These functions are suggested for the all products appearing in the experiments (CO, CO2, H2 and CH3OH). Carbon and methane, which normally are not observed in the reforming products, wereexcluded from consideration. The proposed correction functions are shown in Table 1. Here indexes "cor" and "eq" relate to corrected and equilibrium molar fractions, respectively; CH3OHconv – methanol conversion ratio; T – reforming temperature, oC.
|
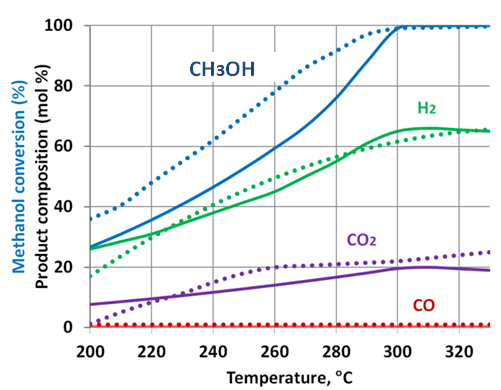 | Figure 4. Comparison of the corrected prediction with experimental data from[30].- theoreticalprediction; ·······- experimental data H2O/CH3OH ratio = 1.3 |
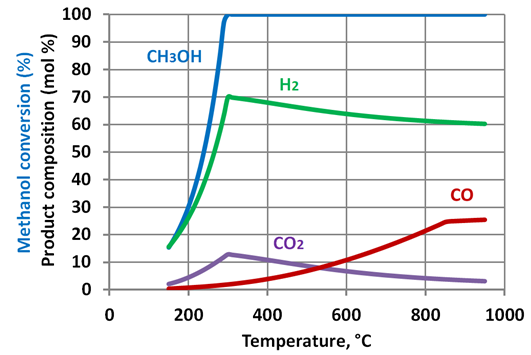 | Figure 5. Methanol reforming products - corrected prediction. Water tomethanol ratio – 0.5:1 |
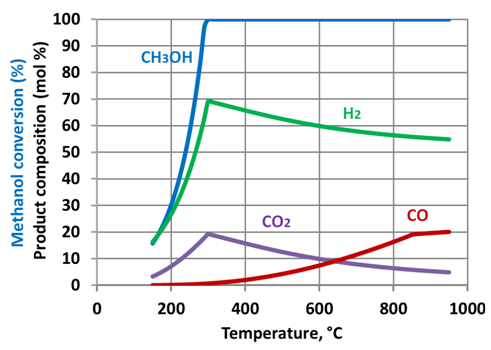 | Figure 6. Methanol reforming products - corrected prediction. Water to methanol ratio – 1:1 |
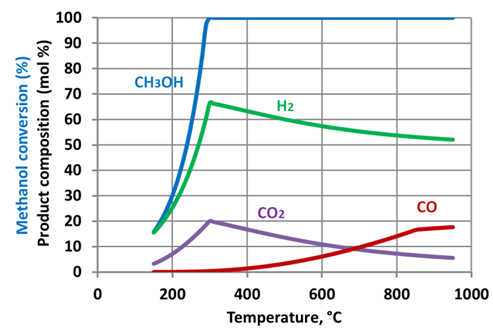 | Figure 7. Methanol reforming products - corrected prediction. Watertomethanol ratio – 1.3:1 |
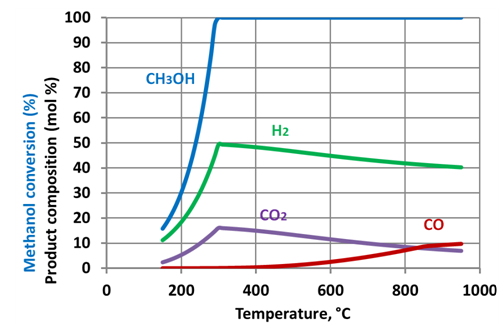 | Figure 8. Methanol reforming products - corrected prediction. Water tomethanol ratio – 3:1 |
3.4. Analysis of Simulation Results
- Simulation results show that increase of the steam to methanol ratio leads to reduction of the H2 and CO yields with an appropriate increase of CO2 formation. Starting from a temperature of circa 300-350°C the methanol is totally converted. As the temperature further increases, the yields of H2 and CO2 reduced. This phenomenon is linked to the reverse water gas shift reaction. The reaction uses hydrogen to produce H2O, and is thermodynamically dominant above 300-350 °C. The prediction results show that maximum hydrogen production can be achieved at a relatively low temperature of approximately 300oC. At the same temperatures full methanol conversion is taking place, as well. The moderate temperature of methanol reforming makes possible utilization of the ICE exhaust gases heat to sustain the endothermic reactions in the reformer.Figure 9 shows the maximal energy efficiency values of methanol steam reforming as a function of the water/alcohol ratio. The required values of the reforming process temperature are shown near corresponding points of the plot. Simulation results showed that the energy efficiency of methanol steam reforming approached maximal value of 0.66 at water to methanol ratio of 1.3 and the reaction temperature of approximately 300oC. For the all considered water to methanolratios (0.5 – 3.0) maximal energy efficiency of the reforming process was observed in the narrow range of temperatures 297-307oC.Comparison of energy efficiency values achievable for methanol steam reforming with those of ethanol[34] showsthat the difference between the maximal values of energy efficiency for these two fuels is not significant. However, for methanol it can be achieved under much lower temperature: 570K compared with 1100K for ethanol. This is a significant benefit of methanol over ethanol in the view of the TCR concept realization.A potential of ICE efficiency improvement and emissions reduction by application of the TCR concept is analysed in[34].
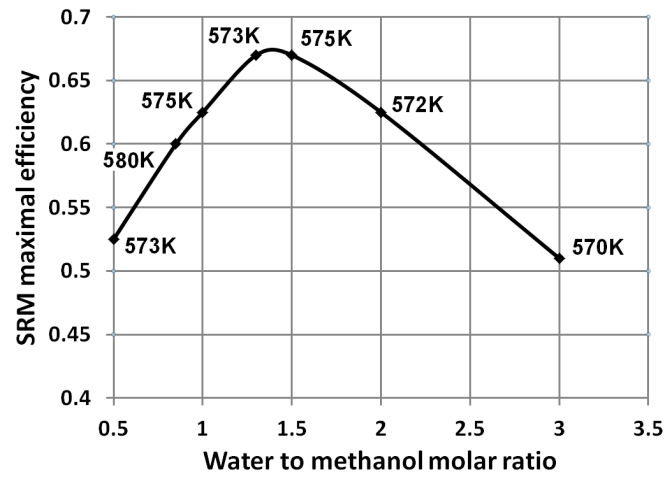 | Figure 9. Energy efficiency of methanol reformingcalculated by eq.(4) |
4. Conclusions
- Modelling and analysis of the methanol steam reforming for the purpose of ICE feeding was performed. The results obtained with the equilibrium simulation model do not agree with the experimental data because this model considers an equilibrium state within an infinite reactor with full methanol conversion.An empiric correlationderived on the base of available experimental datais suggested to bring the simulation results closer to the actual findings. This approach can be useful for analysis of methanol steam reforming when detailed kinetic data are unavailable.The simulation results show that the maximum hydrogen production can be achieved underthe reformingtemperature of approximately 300oC, when full methanol conversion takes place. The moderatetemperature of methanol reforming makes it possible to utilize the ICE exhaust gases heatfor sustaining the endothermic reactions in the reformer.The difference between the maximal achievable values of energy efficiency for methanol and ethanol steam reforming is not significant (0.66 for methanol compared with 0.59 for ethanol). However, for methanol it can be achieved under much lower temperature. This is a significant benefit of methanol over ethanol in the view of the TCR concept realization. This concept can be realized most efficiently in a hybrid propulsion system, where cold start and transient operation problems can be successfully overcome due to availability of additional energy source.
ACKNOWLEDGEMENTS
- Financial support of the Israel Science Foundation, Ministries of Energy & Water Resources and Environmental Protection is highly appreciated. Authors are grateful to Ms. Ron Karasenti and Mr.TamirBengio for their valuable help in calculations carrying out.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML