-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Clinical Medicine and Diagnostics
p-ISSN: 2163-1433 e-ISSN: 2163-1441
2014; 4(1A): 8-14
doi:10.5923/s.cmd.201401.02
Microtubule Based OSM3 Family Kinesin-2 Motors are Involved in Endocytosis in Endothelial Cells
Shahid S. Siddiqui 1, 2, 3, Gias U. Ahmmad 1, 4, Sivakumar Loganathan 1, Fathy M. El-Faskhany 2, 5, Zeba K. Siddiqui 3, Faisal A. Allaf 2, Hanadi A. Lamfoon 2, Mohammad M. Beyari 2
1Section of Pulmonary and Critical Care, Department of Medicine, University of Chicago, Chicago, IL 60637, USA
2Department of Basic and Clinical Oral Sciences, Faculty of Dentistry, Umm Al Qura University, Makkah, Saudi Arabia
3Department of Pharmacology, University of Illinois, Chicago, IL 60612, USA
4Department of Physiology, College of Medicine, Taibah University, Al-Madinah, Saudi Arabia
5Department of Medical Biochemistry, Faculty of Medicine, Tanta University, Tanta, Egypt
Correspondence to: Shahid S. Siddiqui , Section of Pulmonary and Critical Care, Department of Medicine, University of Chicago, Chicago, IL 60637, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Integrity of endothelial monolayer is essential for homeostasis in vasculature. We have previously shown that endocytosis is critical in maintaining the integrity of endothelial monolayer and that 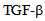 receptor signaling is involved in albumin uptake in pulmonary microvessel endothelial cells. Herein we demonstrate that in microvessel endothelial cells endocytosis is mediated by expression of microtubule based OSM3/Kinesin-2 family anterograde (KIF3A and KIF3B) motors and that albumin endocytosis induces kinesin-2 expression in endothelial cells as shown by immunocytochemistry and immunoblotting. Inhibition of kinesin function attenuates albumin endocytosis. Treatment with
receptor signaling is involved in albumin uptake in pulmonary microvessel endothelial cells. Herein we demonstrate that in microvessel endothelial cells endocytosis is mediated by expression of microtubule based OSM3/Kinesin-2 family anterograde (KIF3A and KIF3B) motors and that albumin endocytosis induces kinesin-2 expression in endothelial cells as shown by immunocytochemistry and immunoblotting. Inhibition of kinesin function attenuates albumin endocytosis. Treatment with 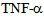 results in the redistribution of kinesin-2 in endothelial cells, and inhibiting
results in the redistribution of kinesin-2 in endothelial cells, and inhibiting 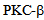 with specific inhibitor Enzastaurin results in reduced response to thrombin induced calcium entry, and albumin endocytosis. In conclusion we have shown the role of kinesin-2 in endocytosis in endothelial cells. These data suggest a novel approach of investigating endothelial barrier function and intracellular trafficking by small molecules that affect kinesin motor function.
with specific inhibitor Enzastaurin results in reduced response to thrombin induced calcium entry, and albumin endocytosis. In conclusion we have shown the role of kinesin-2 in endocytosis in endothelial cells. These data suggest a novel approach of investigating endothelial barrier function and intracellular trafficking by small molecules that affect kinesin motor function.
Keywords:
Endothelial Cells, Endocytosis, Kinesin Motors, 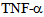 ,
, 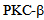 , Enzastaurin
, Enzastaurin
Cite this paper: Shahid S. Siddiqui , Gias U. Ahmmad , Sivakumar Loganathan , Fathy M. El-Faskhany , Zeba K. Siddiqui , Faisal A. Allaf , Hanadi A. Lamfoon , Mohammad M. Beyari , Microtubule Based OSM3 Family Kinesin-2 Motors are Involved in Endocytosis in Endothelial Cells, Clinical Medicine and Diagnostics, Vol. 4 No. 1A, 2014, pp. 8-14. doi: 10.5923/s.cmd.201401.02.
Article Outline
1. Introduction
- Endocytosis is vital for cell survival and signaling. In the endothelial cells lining the vessel wall, serum albumin induces endocytosis; essential for cell growth and endothelial monolayer integrity. A number of proteins have been reported that bind to albumin and have been suggested to mediate albumin endocytosis[1-5]. We have previously shown internalization of the 73-75 kDa
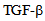 II receptor (TGFBR2), member of the
II receptor (TGFBR2), member of the 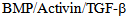 receptor family of kinases[6, 7]. Subsequent to endocytosis intracellular transport of the internalized cargo is fundamental for cellular function, survival and morphogenesis. For example, in corneal endothelium the barrier integrity is maintained by its tight and adherens junctions for the normal function of corneal stroma[8], and this barrier function requires components of both actin and microtubule cytoskeleton, which are coupled to the cell junction through a network of linker proteins[3]. Microtubule based kinesin motors use the hydrolysis of ATP to directionally transport various cargos, such as protein complexes, mRNA, and membranous organelles. Kinesins are highly conserved motors that are conserved in eukaryotes from yeast, worms, flies, and mammals and are involved in intracellular transport of diverse cellular cargo, The complex role of kinesin superfamily has been extensively studied in axonal transport, cytokinesis and chromosomal segregation; their role in intracellular transport in endothelium is not well studied. Since kinesins are highly diverse both structurally and functionally, each kinesin may serve a specific and highly unique function in different cell types. In human placenta, KIF17 kinesin, a member of the OSM3 kinesin has been reported in vascular endothelium in early pregnancy[11]. These authors showed that KIF17, and KIFC1 kinesins have a low to moderate expression in the endothelial cells of villi from normal human-term placentas, but KIF17 and KIFC1expression showed considerably higher in placentas of diabetic and preeclamptic patients[11]. The cytoskeleton dynamics is regulated by Rho family of small GTPases and different protein kinases such as p38 MAPK, several signaling pathways in normal and pathological conditions affect cytoskeleton and thereby endothelial barrier function and integrity[12].
receptor family of kinases[6, 7]. Subsequent to endocytosis intracellular transport of the internalized cargo is fundamental for cellular function, survival and morphogenesis. For example, in corneal endothelium the barrier integrity is maintained by its tight and adherens junctions for the normal function of corneal stroma[8], and this barrier function requires components of both actin and microtubule cytoskeleton, which are coupled to the cell junction through a network of linker proteins[3]. Microtubule based kinesin motors use the hydrolysis of ATP to directionally transport various cargos, such as protein complexes, mRNA, and membranous organelles. Kinesins are highly conserved motors that are conserved in eukaryotes from yeast, worms, flies, and mammals and are involved in intracellular transport of diverse cellular cargo, The complex role of kinesin superfamily has been extensively studied in axonal transport, cytokinesis and chromosomal segregation; their role in intracellular transport in endothelium is not well studied. Since kinesins are highly diverse both structurally and functionally, each kinesin may serve a specific and highly unique function in different cell types. In human placenta, KIF17 kinesin, a member of the OSM3 kinesin has been reported in vascular endothelium in early pregnancy[11]. These authors showed that KIF17, and KIFC1 kinesins have a low to moderate expression in the endothelial cells of villi from normal human-term placentas, but KIF17 and KIFC1expression showed considerably higher in placentas of diabetic and preeclamptic patients[11]. The cytoskeleton dynamics is regulated by Rho family of small GTPases and different protein kinases such as p38 MAPK, several signaling pathways in normal and pathological conditions affect cytoskeleton and thereby endothelial barrier function and integrity[12]. 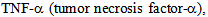 a key cytokine that is associated with inflammatory response triggers induction of p38 MAPK, and causes microtubule disassembly, resulting in compromised barrier function, and intracellular transport[7,12-14]. Here, we have investigated the role of albumin endocytosis in the expression of OSM-3 and related kinesin-2 family motors KIF3A and KIF3B in rat lung microvessel endothelial cells (RLMVEC), and demonstrate that albumin endocytosis induces kinesin-2 and that these motors are redistributed in the endothelial cells following exposure with
a key cytokine that is associated with inflammatory response triggers induction of p38 MAPK, and causes microtubule disassembly, resulting in compromised barrier function, and intracellular transport[7,12-14]. Here, we have investigated the role of albumin endocytosis in the expression of OSM-3 and related kinesin-2 family motors KIF3A and KIF3B in rat lung microvessel endothelial cells (RLMVEC), and demonstrate that albumin endocytosis induces kinesin-2 and that these motors are redistributed in the endothelial cells following exposure with 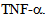 We have also shown that inhibition of protein kinase
We have also shown that inhibition of protein kinase 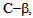 by a small molecule inhibitor enzastaurin affects thrombin induced calcium entry into endothelial cells, providing a new approach in controlling endothelial permeability.
by a small molecule inhibitor enzastaurin affects thrombin induced calcium entry into endothelial cells, providing a new approach in controlling endothelial permeability. 2. Materials & Methods
2.1. Endothelial Cells and Culture Conditions
- Human alpha-thrombin was purchased from Enzyme Research Laboratories (South Bend, Indiana, USA). HUVEC and RLMVEC cells; and growth medium DMEM (Dulbecco’s Modified Eagles Medium), foetal bovine serum (FBS) were obtained from Clonetics Corp. (San Diego, CA). Trypsin and Hanks’ balanced salt solution and (MCDB) media 131 were purchased from (Invitrogen, USA). RLMVEC, or HUVEC cells were grown in DMEM, in 10% FBS or serum free medium supplemented with antibiotics (100 units of penicillin, 100 units of streptomycin) at 37℃ in 5% CO2 as described[6, 14].
2.2. Kinesin-2 (KIF3A, and KIF3B), and OSM3 Antibodies
- OSM3 is a highly conserved member of the kinesin-2 motor family, discovered in Caenorhabditis elegans that is expressed in ciliary chemosensory neurons mediating high salt osmotic avoidance[9,15-18]. It has been shown that OSM3 in C. elegans is homodimeric[8, 19], ¸however, antibodies raised against a conserved C. elegans OSM3 motor domain recognize both kinesin-2 family members KIF3A and KIF3B in lung endothelial cells[our unpublished data]. Antibodies against OSM3 were raised in rabbits and affinity purified that recognized OSM3 protein in C. elegans (Siddiqui, SS, unpublished). Commercial affinity purified polyclonal antibodies against KIF3A and KIF3B were obtained from Santa Cruz Tech, CA, USA.
2.3. Immunoblotting Kinesin-2
- Rat lung microvessel endothelial cells RLMVEC were grown at passage 5-6 in DMEM with 10% FBS, and 100 units of antibiotics as described[6, 14]. RLMVEC were washed twice with chilled at 0℃ phosphate buffered saline (PBS), and whole cell lysates were prepared in RIPA lysis buffer[50 mM Tris (pH 8.0), 150 mM NaCl, 10% glycerol, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS and 0.42% NaF containing protease inhibitor cocktail (1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 5 mg/ml leupeptin)]. The cell lysates were cleared by centrifugation at 10,000 g for 10 minutes. The protein concentrations were determined by using the Bradford assay.Protein lysates (60–80 µg) were separated by 7.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and transferred to PVDF membranes (Millipore, Bedford, MA, USA). The membranes were blocked in 5% non-fat dried milk freshly made in Tris-Buffered Saline Tween-20 (TBST). Affinity purified rabbit polyclonal antibodies to OSM3 were used at 1:1000 dilutions in TBST for 2 hr at room temp, followed by washing in TBST for 10 minutes, twice. Proteins were detected by immunoblotting using an enhanced chemiluminescense kit (NEN Life Science Products, Boston, MA, USA) according to manufacturer’s protocol.
2.4. Albumin Endocytosis, Immunostaining and 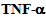 Treatment
Treatment
- RLMVEC were grown on glass cover slips coated with 1% collagen, in DMEM, with serum free medium for 8-12 hours (starvation conditions) as previously described[6, 14]. Cells were washed with PBS, and fixed in 4% freshly prepared paraformaldehyde in PBS for 10 minutes, washed with PBS, an made permeable with chilled methanol for 10 minutes, washed with TPBS (PBS with 0.1% Triton X-100), twice. Cells were incubated in Kinesin-2 antibodies (KIF3A or KIF3B, Santa Cruz Tech. CA) primary antibodies (diluted 1: 500 in TPBS), for 1 hour at 4 C, washed twice with TPBS and incubated with fluorescent conjugated secondary anti-rabbit IgG antibodies (diluted 1: 250 in TPBS) for 30 minutes. Cells were washed and mounted in 5% n-propyl gallate in glycerol to prevent bleaching; observed under a Nikon Eclipse fluorescent microscope equipped with appropriate ultraviolet light excitation and barrier filters for observing Alex-480, or Alexa-560 conjugated secondary antibodies. TNF-∝ was purchased from R & D Systems (USA). RLMVEC cells were starved for 24 hours and grown in DMEM, serum free medium at 37 C in 5% CO2 as described[6, 14]. Cells were treated as described above, starved for 6-8 hours and incubated with 10 ng of the cytokine.
2.5. [Ca2+]i Measurements in Endothelial Cells
- An increase in[Ca2+]i was measured using the Ca2+ sensitive fluorescent dye Fura 2/AM as described[7,19,20]. For loading of cells with Fura 2/AM, cells grown on 25-mm coverslips were incubated with 3 uM Fura 2 for 15 min at 37°C. RLMVEC grown in 5-6 passage were used. RLMVEC were washed twice with Hanks’ balanced salt solution and imaged using an Attoflor Ratio Vision digital fluorescence microscopy system (Atto Instruments, Inc., Rockville, MD) equipped with a Zeiss Axiovert S100 inverted microscope and an F-Fluar_40× magnification, 1.3-numerical aperture oil immersion objective. Regions of interest in individual cells were marked and excited at 334 and 380 nm with emission at 520 nm. The 334/380 nm excitation ratio, which increased as a function of[Ca2+]i, was captured at 5-s intervals.
2.6. Treatment of Endothelial Cells with PKC Inhibitor Enzastaurin
- RLMVEC were treated to varying concentrations of enzastaurin, a PKC-beta inhibitor for the indicated time periods as described[21].
2.7. Statistical Analysis
- Each experiment was performed three times, independently. For comparisons of frequencies among different categories, the Fischer’s exact test was used.
3. Results
3.1. Kinesin-2 family members, KIF3A and KIF3 B Express in Endothelial Cells
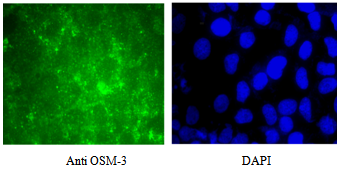
- OSM3 is a highly conserved member of the kinesin-2 motor family, discovered in Caenorhabditis elegans that is expressed in ciliary chemosensory neurons mediating high salt osmotic shock[9,13,15-17]. We have used antibodies raised against OSM3 protein to detect kinesin-2 motor proteins in RLMVEC. OSM3 antibodies recognize two members of the kinesin-2 family, KIF3A and KIF3B. These results are confirmed by immunoblotting with KIF3A and KIF3B antibodies (Santa Cruz Tech. CA); SSS: unpublished data. Figure 1 shows that RLMVEC express both KIF3A and KIF3 B members of the kinesin-2 family[10,12].
3.2. Expression of OSM3 Kinesin-2 Motors KIF3A & KIF3B in HUVEC and RLMVEC Endothelial Cells
- We examined whether KIF3A and KIF3B antibodies stain different types of endothelial cells, and stained human umbilical vascular endothelial cells, grown at passage 4-5.
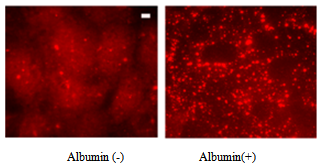
3.3. Albumin Endocytosis induces Kinesin-2 Expression
- We investigated the role of kinesin-2 motors in endocytosis of albumin in endothelial monolayer; as endocytosis is critical for cell proliferation, growth and homeostasis[6,7,19]. In RLMVEC which have been grown in serum free medium in starvation conditions for 8-12 hours, addition of serum albumin induces endocytosis[6, 14]. Figure 3, shows that albumin endocytosis induces expression of KIF3B motors (both KIF3A and KIF3B, showed similar staining pattern). Highly punctate expression of kinesin-2 motors in the cell cytoplasm and cell surface is clearly observed.
3.4. Higher Expression of Kinesin-2 in Cell Junction Region following Albumin Endocytosis
- Kinesin-2 is an anterograde motor that consists of two heteromeric KIF3A and KIF3B motors and an associated KAP-1 non-motor protein that is required to attach cellular cargo to the microtubule based kinesin-2 motors[9,10,12,13, 22,23]. Interestingly, Kinesin-2 motors (KIF3A and KIF3B) also express in growing cell contacts and in plasma-lemma, and in membranes that may form tight and adherens junctions[19], between endothelial cells (Figure 4). In the starved endothelial cells, kinesin-2 motors are primarily expressed in the perinuclear region and relatively small expression is seen in the cell surface.
3.5. 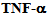 Treatment Causes Redistribution of Kinesin-2
Treatment Causes Redistribution of Kinesin-2
- The inflammatory cytokine
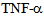 is known to stimulate c-Jun N terminal kinase (JNK) in neurites[10,12,24]; causing kinesin heavy chain family KIF5B motor protein to dissociate from tubulin in axons but not from cell bodies as deduced from Forster resonance energy transfer (FRET) analysis. We have examined the distribution of kinesin-2 (KIF3B) following stimulation by
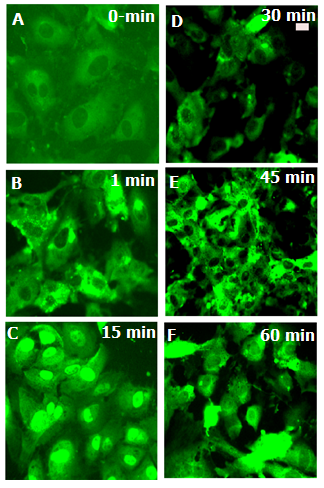
is known to stimulate c-Jun N terminal kinase (JNK) in neurites[10,12,24]; causing kinesin heavy chain family KIF5B motor protein to dissociate from tubulin in axons but not from cell bodies as deduced from Forster resonance energy transfer (FRET) analysis. We have examined the distribution of kinesin-2 (KIF3B) following stimulation by 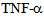 (Figure 4) and observed translocation of KIF3B within 1-15 minutes of cytokine exposure. There is a dynamic change in kinesin-2 (KIF3B) expression and redistribution of the motor proteins from cell membrane and cytosol to perinuclear region within 15 minutes (Figure 4). This is followed b a return to cytosolic expression in 45 minutes; the monolayer appears to show cellular gaps, as shown in Figure 4. After one hour of the TNF-∝ treatment, the kinesin-2 expression returns to lower levels, however the integrity of the endothelial monolayer is reduced.
(Figure 4) and observed translocation of KIF3B within 1-15 minutes of cytokine exposure. There is a dynamic change in kinesin-2 (KIF3B) expression and redistribution of the motor proteins from cell membrane and cytosol to perinuclear region within 15 minutes (Figure 4). This is followed b a return to cytosolic expression in 45 minutes; the monolayer appears to show cellular gaps, as shown in Figure 4. After one hour of the TNF-∝ treatment, the kinesin-2 expression returns to lower levels, however the integrity of the endothelial monolayer is reduced.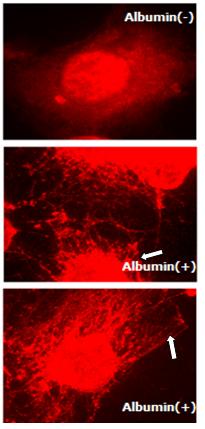 | Figure 4. Kinesin-2 (KIF3B) motors express in the growth cones, filopodia of growing endothelial cells, following albumin endocytosis. The white arrow shows KIF3B kinesin staining in the filopodia |
3.6. Regulation of Store-operated Ca+ Entry by Kinesin Specific Inhibitor
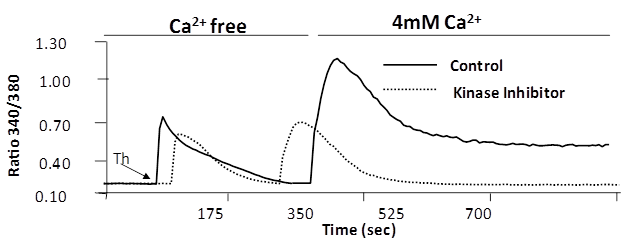
- Regulation of calcium ion in endothelial cells, intrinsically and extrinsically is important for maintaining the barrier integrity[3,19,20]. This dynamic equilibrium has been studied by challenging endothelial monolayer with thrombin, and measuring calcium ion modulation by Fura-2 dye[7,19, 20]. In Fura 2-loaded RLMVEC, the thrombin response consists of an immediate but transient rise in intracellular Ca2+ due to release of sequestered Ca2+ from the endoplasmic reticulum (ER), followed by a secondary rise in intracellular Ca2+ due to Ca2+ entry. We experimentally separated the two phases using a Ca2+ add-back protocol (Figure 6). When administered under Ca2+-free bath conditions, thrombin elicited only the Ca2+ transient due to release of sequestered Ca2+ into the cytoplasm. A more sustained Ca2+ signal appeared subsequently when extracellular Ca2+ was restored to the bath medium, signifying entry of extracellular Ca2+ into cells. We observed the blocking effect of a PKC-β specific inhibitor, enzastaurin on Ca2+ entry via store operated channels (SOCs). The marked inhibitory action of enzastaurin on Ca2+ entry was evident because the Ca2+ release from endoplasmic reticulum (ER) stores was similar in control and treated cells (Figure 6).
4. Discussion
- Highly diverse and specific roles of different members of microtubule based kinesin and dynein motor proteins have revealed that the members of kinesin superfamily are not only different in their structures such as the location of motor domain, length of non-motor tail region and associated proteins, but also the cellular cargo they carry during intracellular transport on microtubules[9,10,12]. However the role and cellular cargo of kinesins in endothelial cells is poorly investigated. Our data demonstrate (Figure 1, Figure 2) that members of the kinesin-2 family are expressed in rat lung microvessel endothelial cells both at mRNA (data not shown), and protein expression levels. We have demonstrated that onset of endocytosis in pulmonary endothelial cells, such as that initiated by albumin uptake in serum starved endothelial cells also induces kinesin-2 motor expression (Figure 3). In a non-confluent endothelial monolayer culture, albumin endocytosis results in kinesin-2 expression not only in the cell cytoplasm, but remarkably also in the growth cones and plasma-lemma and membranes. These fibre like protrusions and cell extensions may form tight and adherens junctions, and help in the formation and assembly of the endothelial monolayer (Figure 3). Our results at light microscopic level cannot fully ascertain the role of kinesin-2 in cell junction formation, but electron microscopy of such endothelial monolayers could shed light on the role of kinesin-2 in junction formation in pulmonary endothelial cells. Our data also suggest that treating RLMVEC with
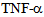 causes redistribution of kinesin-2 motors in a time dependent manner, and this new localization of motors could play a part in cytokine mediated signaling. Future experiments using cellular and biochemical assays could reveal the role of kinesin motors in inflammatory cytokine signal transduction[14,19]. Inflammatory cytokines such as
causes redistribution of kinesin-2 motors in a time dependent manner, and this new localization of motors could play a part in cytokine mediated signaling. Future experiments using cellular and biochemical assays could reveal the role of kinesin motors in inflammatory cytokine signal transduction[14,19]. Inflammatory cytokines such as 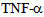 are known to disrupt microtubule and actin cytsoskeletal organization, causing disruption of endothelial barrier function[10,12]. Previously MAP kinase kinase kinase MLK2 has been shown to interact with activated JNK along microtubules and with OSM3 ortholog KIF3[12], using a yeast hybrid approach. These authors also showed association of MLK2 with KAP3 a non-motor associated component of the KIF3 kinesin-2 complex. Figure 5 shows that untreated control endothelial cells express a low to moderate level of kinesin-2, but exposure to
are known to disrupt microtubule and actin cytsoskeletal organization, causing disruption of endothelial barrier function[10,12]. Previously MAP kinase kinase kinase MLK2 has been shown to interact with activated JNK along microtubules and with OSM3 ortholog KIF3[12], using a yeast hybrid approach. These authors also showed association of MLK2 with KAP3 a non-motor associated component of the KIF3 kinesin-2 complex. Figure 5 shows that untreated control endothelial cells express a low to moderate level of kinesin-2, but exposure to 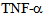 induces kinesin-2 expression within a minute and in 15 minutes kinesin-2 appears to be highly expressed in perinuclear region as compared to the cell membrane and cytosolic regions, suggesting a dynamic change in microtubule based cytoskeleton and kinesin-2 localization. In 45 minutes there is a return to cytosolic expression and the monolayer appears to show cellular gaps. In one hour the kinesin-2 expression returns to lower levels, however the integrity is lost and the treated endothelial monolayer shows gaps. These data indicate a dynamic role of kinesin-2 motors in inflammatory cytokine response by endothelial cells. The role of MAP kinases such as p38 in the
induces kinesin-2 expression within a minute and in 15 minutes kinesin-2 appears to be highly expressed in perinuclear region as compared to the cell membrane and cytosolic regions, suggesting a dynamic change in microtubule based cytoskeleton and kinesin-2 localization. In 45 minutes there is a return to cytosolic expression and the monolayer appears to show cellular gaps. In one hour the kinesin-2 expression returns to lower levels, however the integrity is lost and the treated endothelial monolayer shows gaps. These data indicate a dynamic role of kinesin-2 motors in inflammatory cytokine response by endothelial cells. The role of MAP kinases such as p38 in the 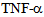 induced disintegration of microtubule assembly, via rho GTPases have been reported[12,14,18,21,25, 26]; as p38 MAPK can trigger the reactive oxygen species (ROS) production and induction of Hsp27 (heat shock protein 27),[18]. ROS have been reported to interfere with microtubule assembly. It has been shown that
induced disintegration of microtubule assembly, via rho GTPases have been reported[12,14,18,21,25, 26]; as p38 MAPK can trigger the reactive oxygen species (ROS) production and induction of Hsp27 (heat shock protein 27),[18]. ROS have been reported to interfere with microtubule assembly. It has been shown that 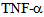 induces activation of p38 MAPK, and that p38 specific inhibitors such as SB 203580 can suppress the loss in trans-endothelial resistance (TER), an indicator of barrier integrity[14, 29, 30, 31]. Further experiments using transcytosis assays[3], could establish the role of kinesin-2, by using kinesin-2 specific siRNA or inhibitors, in the maintenance of monolayer barrier function by influencing the cytoskeleton dynamics. Thus, inhibition of
induces activation of p38 MAPK, and that p38 specific inhibitors such as SB 203580 can suppress the loss in trans-endothelial resistance (TER), an indicator of barrier integrity[14, 29, 30, 31]. Further experiments using transcytosis assays[3], could establish the role of kinesin-2, by using kinesin-2 specific siRNA or inhibitors, in the maintenance of monolayer barrier function by influencing the cytoskeleton dynamics. Thus, inhibition of 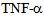 mediated signalling could prevent barrier leakage and disintegration of endothelial monolayer. One such small molecular intervention may be examined through Enzastaurin that inhibits
mediated signalling could prevent barrier leakage and disintegration of endothelial monolayer. One such small molecular intervention may be examined through Enzastaurin that inhibits 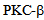 [18], and attenuates calcium entry after endothelial cells are challenged with Thrombin. Figure 5 shows that pre-treatment of RLMVEC with the
[18], and attenuates calcium entry after endothelial cells are challenged with Thrombin. Figure 5 shows that pre-treatment of RLMVEC with the 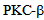 inhibitor enzastaurin could inhibit the calcium entry into the cells (Figure 6). RLMVEC monolayers undergo an immediate increase in intracellular calcium, following thrombin treatment that can be visualized by Fura 2 imaging. This increase in calcium is for a brief interval as calcium is released from the endoplasmic reticulum calcium stores, which are followed by a secondary increase in intracellular calcium caused by the entry of calcium from extra-cellular medium[7]. These two distinct phases have been separated in the experiments described in Figure 5, using a Ca2+add-back protocol. If the extracellular medium in the bath is kept calcium free, that allows thrombin induced Ca2+ transient due to the sequestered Ca2+ in the cytoplasm. When calcium is available in the extracellular medium, a more sustained Ca2+ signal appears later, suggesting that calcium enters from outside the cell into the cell. Interestingly, the PKC-beta specific inhibitor enzastaurin blocks the entry of calcium via the store operated channels (SOCs). Our data in Figure 5 shows that enzastaurin inhibition on Ca2+ entry was pronounced as the calcium release from the ER stores was very similar in the inhibitor treated cells and in control cells. These results suggest that
inhibitor enzastaurin could inhibit the calcium entry into the cells (Figure 6). RLMVEC monolayers undergo an immediate increase in intracellular calcium, following thrombin treatment that can be visualized by Fura 2 imaging. This increase in calcium is for a brief interval as calcium is released from the endoplasmic reticulum calcium stores, which are followed by a secondary increase in intracellular calcium caused by the entry of calcium from extra-cellular medium[7]. These two distinct phases have been separated in the experiments described in Figure 5, using a Ca2+add-back protocol. If the extracellular medium in the bath is kept calcium free, that allows thrombin induced Ca2+ transient due to the sequestered Ca2+ in the cytoplasm. When calcium is available in the extracellular medium, a more sustained Ca2+ signal appears later, suggesting that calcium enters from outside the cell into the cell. Interestingly, the PKC-beta specific inhibitor enzastaurin blocks the entry of calcium via the store operated channels (SOCs). Our data in Figure 5 shows that enzastaurin inhibition on Ca2+ entry was pronounced as the calcium release from the ER stores was very similar in the inhibitor treated cells and in control cells. These results suggest that 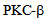 inhibitors such as enzastaurin can be novel therapeutic reagents for regulating the calcium entry and cytsoskeletal dynamics in lung endothelial cells. Using special drug delivery methods employing tissue specific liposome preparations could allow small molecules such as enzastaurin and microRNA reagents such as mimics and antagomirs in pharmacological studies in both in vitro and in vivo models[17]
inhibitors such as enzastaurin can be novel therapeutic reagents for regulating the calcium entry and cytsoskeletal dynamics in lung endothelial cells. Using special drug delivery methods employing tissue specific liposome preparations could allow small molecules such as enzastaurin and microRNA reagents such as mimics and antagomirs in pharmacological studies in both in vitro and in vivo models[17]5. Conclusions
- Endothelial cells are highly specialized cells that function in diverse tissues such as umbilical cord, pulmonary vascular barrier, corneal endothelium and periodontal tissue[3, 6, 8, 28]. We conclude that lung endothelial cells express members of the kinesin-2 family motor proteins (KIF3A and KIF3B) that facilitate albumin endocytosis. The two KIF3A and KIF3B motor proteins are translocated in endothelial cells following exposure to the inflammatory cytokine
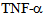 and signaling stimulus.We further show that
and signaling stimulus.We further show that 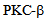 kinase plays a role in kinesin-2 dynamics and inhibition of
kinase plays a role in kinesin-2 dynamics and inhibition of 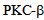 with a specific inhibitor enzastaurin decreases calcium entry, following thrombin mediated calcium entry. These data provide a novel approach in lung endothelial monolayer physiology to investigate the role molecular motors in endocytosis, maintenance of barrier integrity and vascular homeostasis. Enzastaurin can also be examined as a therapeutic target for endothelial function. Further studies are needed to reveal the cellular cargo of kinesin-2 motors in endothelial cells, and the role of individual motors KIF3A and KIF3B, and the kinesin-2 associated protein KAP-1[10, 20]
with a specific inhibitor enzastaurin decreases calcium entry, following thrombin mediated calcium entry. These data provide a novel approach in lung endothelial monolayer physiology to investigate the role molecular motors in endocytosis, maintenance of barrier integrity and vascular homeostasis. Enzastaurin can also be examined as a therapeutic target for endothelial function. Further studies are needed to reveal the cellular cargo of kinesin-2 motors in endothelial cells, and the role of individual motors KIF3A and KIF3B, and the kinesin-2 associated protein KAP-1[10, 20]ACKNOWLEDGEMENTS
- We thank Distinguished Professor and Head of Pharmacology Department, Dr. A. B. Malik for his support at the University of Illinois, Chicago. We also thank Drs. R. Minshall, C. Tiruppathi, D. Mehta, Oscar Colaminici, Douglas Yau, Richard Ye, A. Rahman, S. Uddin, for discussions and cooperation at UIC. We thank Prof. E. Vokes, Chairman Department of Medicine, University of Chicago, Chicago, IL for his encouragement. We also thank Dr. Sohail Bin Salim BaJammal, Vice Dean for Research and Graduate Studies, Umm Al Qura University, Saudi Arabia. Fathy M. El-Fasakhany is on secondment, Tanta University Faculty of Medicine, Medical Biochemistry Department, Tanta, Egypt.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML