Víctor Hugo Flores Flores , Jesús Cerezo Román , Gisela Montero Alpírez
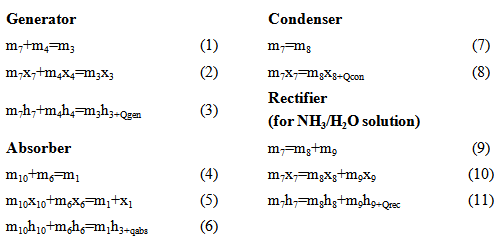
Instituto de ingeniería de la Autónoma de Baja California, Blvd Benito Juárez s/n, Mexicali, Baja California, CP 21280, México
Correspondence to: Jesús Cerezo Román , Instituto de ingeniería de la Autónoma de Baja California, Blvd Benito Juárez s/n, Mexicali, Baja California, CP 21280, México.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Absorption refrigeration systems have been subject of study, because they mainly use thermal energy to function; the fluids conventionally used (NH3-H2O and H2O-LiBr) have low efficiencies. This work analyses six different working fluids (H2O/LiBr, NH3/H2O, NH3/NaSCN, NH3/LiNO3, H2O/LiCl and H2O/CaCl2); and their thermodynamic and energetic characteristics are stablished with the objective of analyzing the system efficiency, as well as its thermophysical properties. For the parametric analysis, a mathematical model based on the first law of thermodynamics was developed. Results showed that H2O/LiCl and H2O/LiBr showed the higher coefficient of performance (COP) with a value of 0.8, operating at A/C conditions.
Keywords:
Absorption refrigeration cycle, Efficiency, Thermodynamic, NH3-H2O y H2O-LiBr, H2O/LiBr, NH3/H2O, NH3/NaSCN, NH3/LiNO3, H2O/LiCl y H2O/CaCl2
Cite this paper: Víctor Hugo Flores Flores , Jesús Cerezo Román , Gisela Montero Alpires , Performance Analysis of Different Working Fluids for an Absorption Refrigeration Cycle, American Journal of Environmental Engineering, Vol. 4 No. 4A, 2014, pp. 1-10. doi: 10.5923/s.ajee.201401.01.
1. Introduction
In places with extreme weather conditions as the north of the Mexican Republic, the consumption of electricity is very high, especially in summer. This has caused a serious problem, not only environmental, but social and economic, principally because of the use of air-conditioning and refrigeration systems are based on electric energy consumption. In the particular case of the city of Mexicali, residential consumption represents nearly 40% of annual total consumption of electric energy due to air-conditioning systems. Absorption cooling systems have the advantage of consuming a small quantity of electric energy, in comparison to vapor compression systems, because of the possibility of using solar energy to apply heat in the generator [1], what has been of interest to the technological community. Working fluids H2O/LiBr and NH3/H2O are the most conventionally used in absorption cooling systems, due to their excellent thermic properties that make them capable to be used in cooling systems at commercial level. However, the disadvantage that has in the case of H2O/LiBr is that the working fluid presents risk of crystallization on concentrations over 68% and cannot work at A/C conditions. On the other hand, NH3/H2O showed a low efficiency for the system. This work Analyses the study of the proposes for the working fluids NH3/LiNO3, NH3/NaSCN, H2O/LiCl and H2O/CaCl2 with the purpose of analyzing their thermophysical characteristics and their working conditions for refrigeration as an alternative for this system.
2. Methodology
The software Engineering Equation Solver (EES) was used as a tool for computer simulation, using the properties of working fluids that the database of the software and the biographical references [5] provided, being applied to a simple absorption refrigeration cycle [4] for application of air-conditioning. The efficiency of the solutions was compared, as well as their thermodynamic characteristics, based on the results of the simulation. The energy balance was obtained using the diagram for a simple absorption refrigeration system shown in figure 1, which gives us the mathematical model used in the software.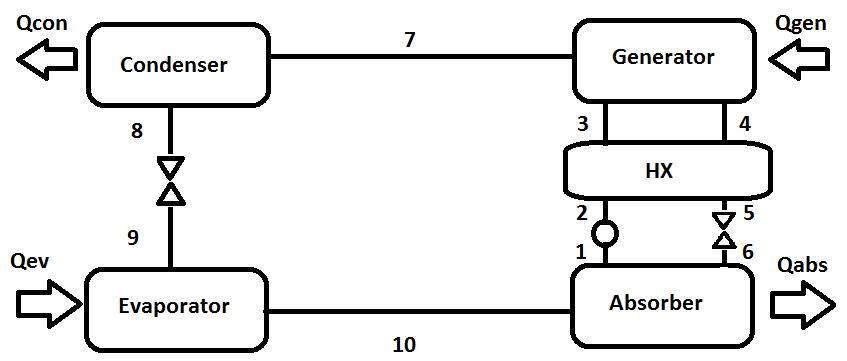 | Figure 1. Diagram of a simple absorption refrigeration system |
3. Results
The SSAS was simulated at A/C conditions: Condenser temperature = 40°C, evaporator temperature = 10°C, absorber temperature = 35°C, the temperature of the generator was varied from 60 to 120 C. The heat exchanger and solution pump have a efficiency of 85%.Figures 2 and 3 show the COP in function of the generator temperature and the mass fraction, respectively. Graphics illustrate that all working fluids show a behavior of stabilization over 100ºC; at the same time, H2O/LiCl and H2O/LiBr present a small range of operation due to crystallization problems. Figure 3 shows that the mixture at previously established work conditions H2O/CaCl2 operates in a short range of lower concentration %X = 0.05, while the mixture NH3-LiNO3 obtains a value of %X = 0.2.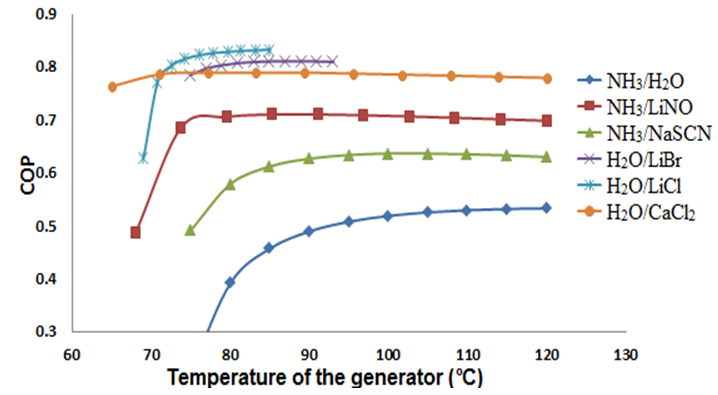 | Figure 2. Relation between the generator temperature (°C) and the COP of the system |
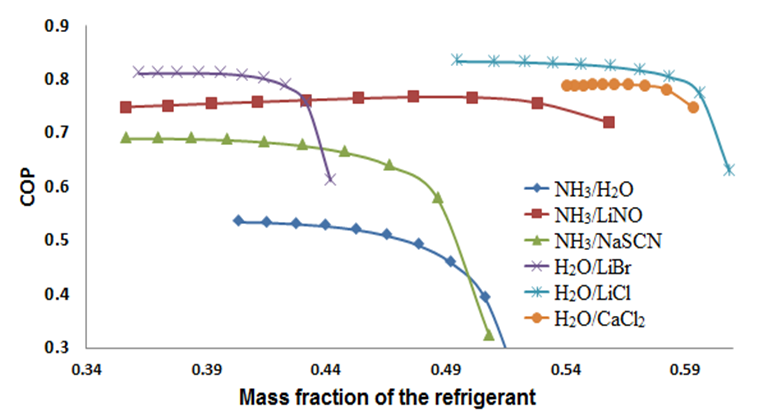 | Figure 3. Relation between the COP and the mass fraction of the refrigerant |
Figures 4, 5 and 6 show the density, viscosity and specific heat as function of the refrigerant mass fraction. The density of each fluid decreases when the mass fraction increases, as shown in figure 4. H2O-LiBr is the working fluid with the highest density with a value of around 1,700 kg/m3. Figure 5 shows that each working fluid has different behavior while increasing the mass concentration and the generator temperature. 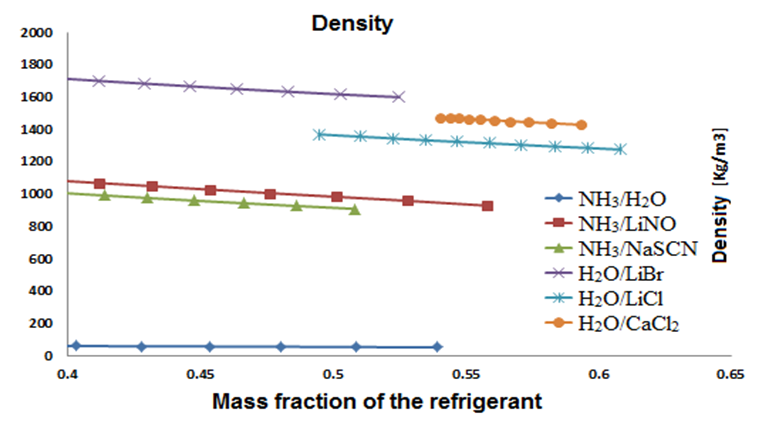 | Figure 4. Relation between density and the concentration of refrigerant |
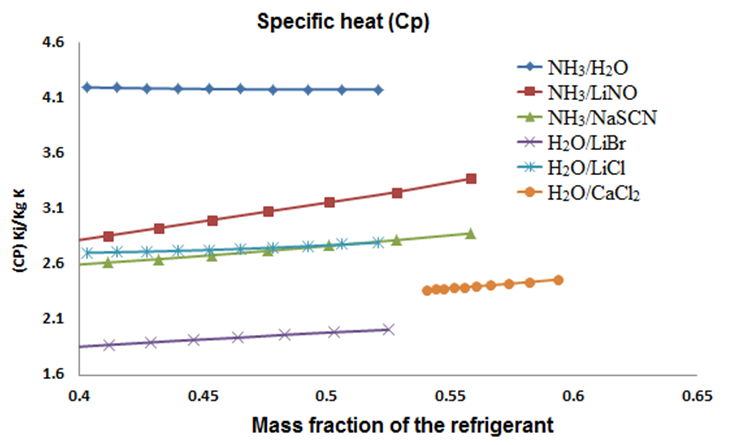 | Figure 5. Relation between viscosity (kg/m s) and the concentration of refrigerant (%) |
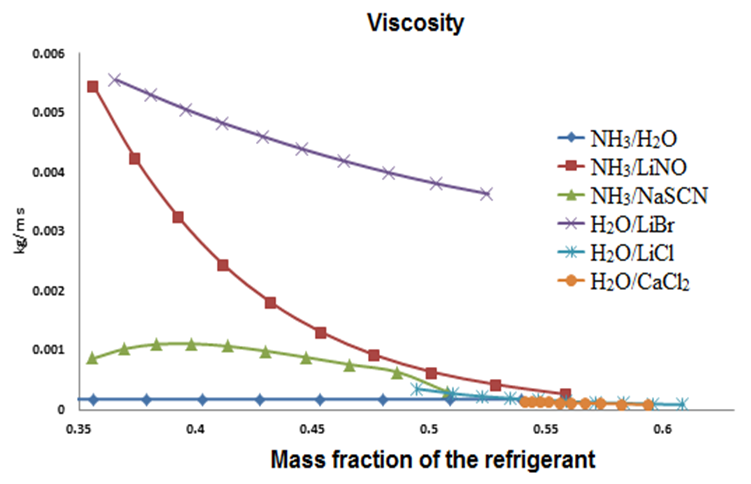 | Figure 6. Relation of heat capacity to the concentration of refrigerant |
The H2O-LiBr mixture had the highest viscosity (0.006 – 0.004 kg/m s), while the lowest was for the NH3-H2O mixture. The concentration’s effect and the generator’s temperature had a same effect on the NH3-H2O, H2O/LiCl and H2O/CaCl2 mixtures. An exception was NH3/NaSCN fluid, which showed a maximum of 0.96 kg/m s and then diminished throughout its range; at the same time the NH3/LiNO3 showed a significant change in its operating range going from 0.0055 to less than 0.001 kh/m-s.In figure 6, the specific heat (Cp) of each fluid shows a high value for NH3/H2O; unlike H2O/LiBr that shows a low Cp for which requires less energy to increase its temperature.
3.1. Crystallization analysis of Work Fluids
Crystallization range of each work fluid depends on the operating temperature. As can be seen in Figure 2, H2O/LiBr crystallizes at a concentration higher than 68% in absorbent weight, its operation range can be appreciated in Figure 7.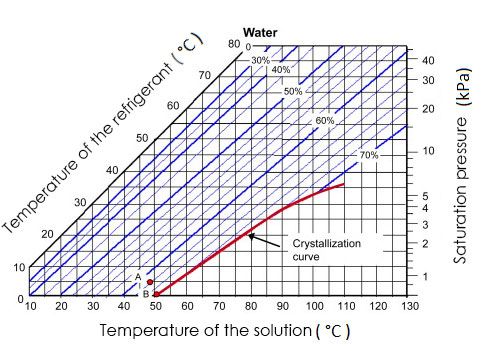 | Figure 7. Crystallization curve of H2O/LiBr [7] |
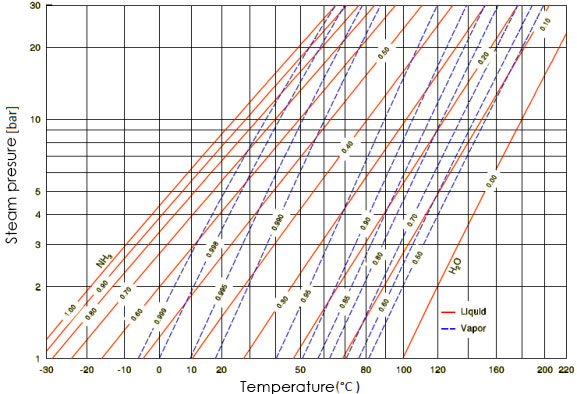 | Figure 8. Temperature and pressure of NH3/H2O [6] |
Figures 9 and 10 show crystallization curves of NH3-LiNO3 mixture in function with concentration and temperature. Both fluids have the advantage to operate below 0°C in a cooling system. However, they have the risk of crystallization, as Figure 9 shows the NH3-LiNO3 mixture crystallizes at concentrations below the 25% of mass fraction [2]. While the NH3/NaSCN mixture has the risk of crystallization at concentrations below 35% of mass fraction [2], as can be seen in Figure 10.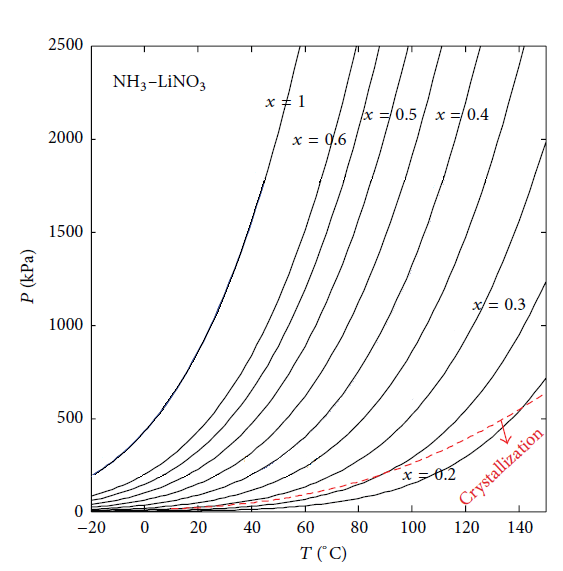 | Figure 9. Crystallization curve of NH3/LiNO3 |
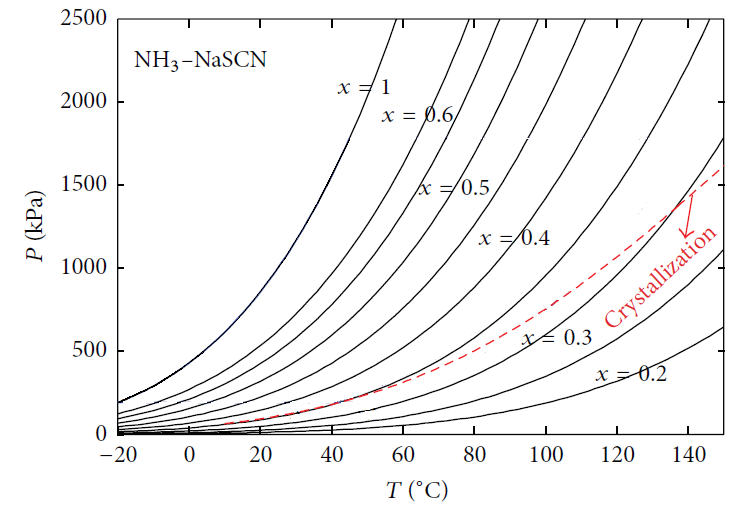 | Figure 10. Crystallization curve of NH3/NaSCN |
In Figure 3, H2O/LiCl and H2O/CaCl2 show a short range of operation in comparison to the rest of the work fluids. Due that they work, respectively, in ranges of 39 to 50% and 40 to 45% at 40°C in the absorber. This is because in their range exists the risk of crystallization at these operation conditions, as we can observe in Figure 11 and 12. 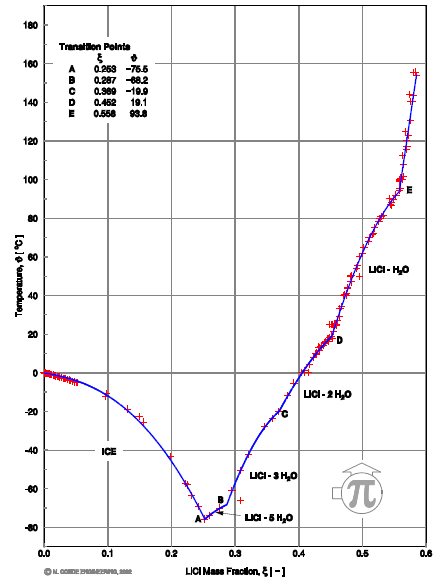 | Figure 11. Crystallization curve of H2O/CaCl2 [5] |
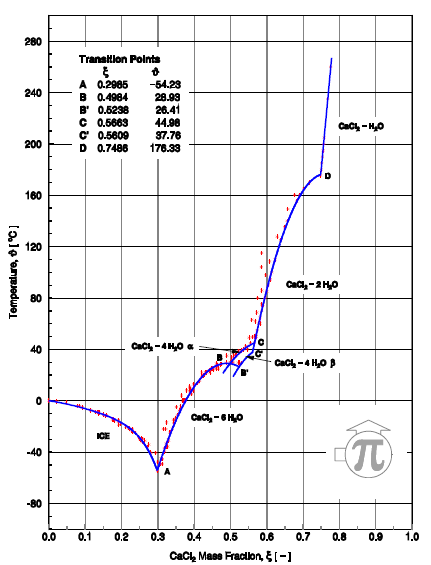 | Figure 12. Crystallization curve of H2O/LiCl [5] |
In table 1, some of the thermodynamic characteristics of each working fluid in AC conditions are shown. The viscosity of H2O/LiBr is at least 4 times higher than other work fluids studied, meaning that the mass transference of the refrigerant in the solution in the absorber would be difficult.| Table 1. Thermodynamic characteristics based of maximum COP |
| | | COP | T (°C) | X %weight | Density kg/m3 | Viscosity, Kg/m-s | Cp kJ/kg °C | | H2O/LiCl | 0.83 | 85.0 | 49.48 | 1368.0 | 0.350 | 2.70 | | H2O/LiBr | 0.81 | 85.0 | 39.60 | 1682.0 | 4.598 | 1.89 | | H2O/CaCl2 | 0.79 | 89.4 | 54.74 | 1464.0 | 0.147 | 2.38 | | NH3/LiNO3 | 0.71 | 91.1 | 45.35 | 1027.0 | 1.307 | 2.99 | | NH3/NaSCN | 0.63 | 105.0 | 39.85 | 1006.0 | 1.111 | 2.61 | | NH3/H2O | 0.53 | 120.0 | 31.23 | 64.2 | 0.174 | 4.19 |
|
|
3.2. Analysis of the Components
In Figure 13, the COP behavior at the change of the evaporator temperature can be observed, being less efficient at the necessity of lower temperature. The working fluids that use water as refrigerant can’t work below 0°C, since water freezes at that temperature and the solutions H2O/CaCl2 and NH3/LiNO3 show a similar behavior.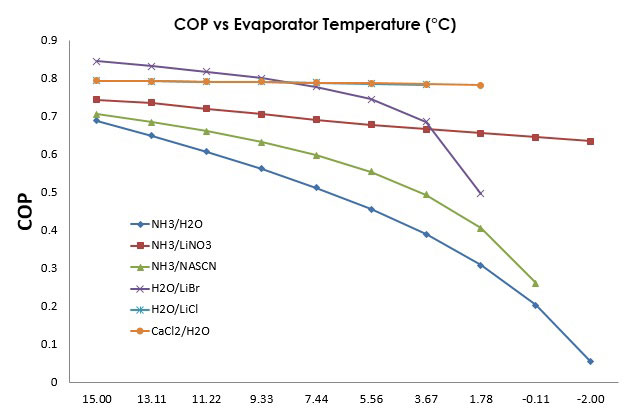 | Figure 13. Relation between the evaporator Temperature and the COP of the system |
In Figure 14 can be seen that with the decreasing of the absorber temperature the COP increases, which helps the refrigerant absorption. The solutions H2O/CaCl2 and NH3/LiNO3 show a similar behavior, being part of the most efficient of the system.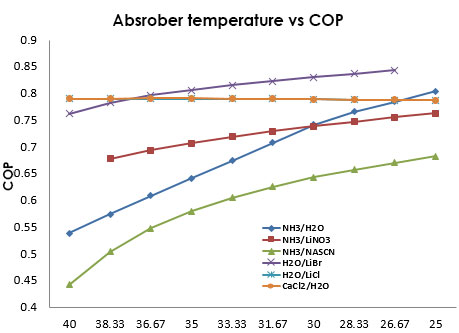 | Figure 14. Relation between the absorber Temperature and the COP of the system |
In Figure 15, the thermal load of each component can be observed, The solution NH3/H2O is the one that has the highest thermal load over 2 kW and the solutions with less heat flow are the ones that have water as refrigerant.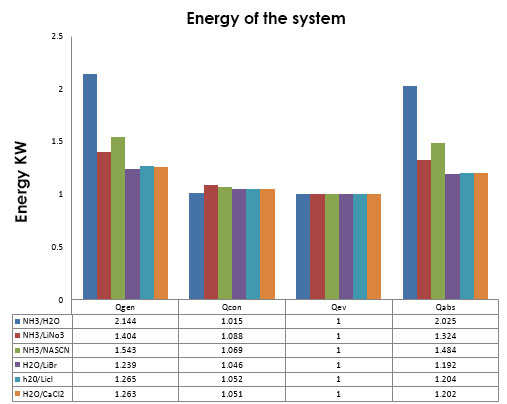 | Figure 15. Heat flow of the components of the system |
In figure 16 the COP of each working fluid in a absorption refrigeration cycle can ve observed at the same working conditions (Condenser temperature = 40°C, evaporator temperature = 10°C, absorber temperature = 35°C and generator temperature =80 °C), the most efficient working fluid at these conditions is H2O/LiBr with a COP above 0.8 flowed by H2O/LiCl and H2O/CaCl2 with a COP aof 0.79. The solution NH3/H2O is the most inefficient working fluid of the six with a COP less than 0.5.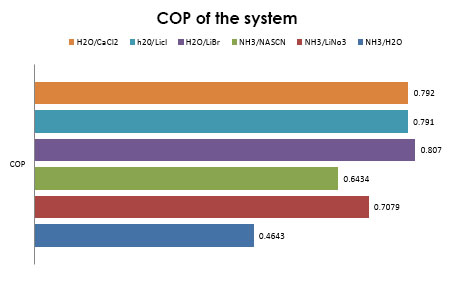 | Figure 16. System COP of each solution |
4. Conclusions
A simulation of a simple cooling cycle with six different work fluids was carried out, in which the less efficient fluid was NH3/H2O with a COP of 0.53. One of the causes of NH3/H2O low efficiency is because of the need of a rectifier, its high cp of 4.17 kg kJ/kg °C, and it had the lowest viscosity and density of all. H2O/LiBr and H2O/LiCl solutions had the highest COP of all the evaluated solutions (around 0.81) because they have a lower cp using less energy for its performing. NH3/LiNO3 y NH3/NaSCN solutions show a similar behavior respectively. However, NH3/LiNO3 is more efficient with a COP of 0.71, in contrast to NH3/NaSCN with a COP of 0.63. Both had similar density but a difference in their Cp which can be a less value of COP for this work fluid. H2O/CaCl2 solution shows a lower Cp after H2O/LiBr solution, which gives one of the causes the makes it efficient. Since H2O/LiBr solution doesn’t require a great amount of energy to increase its temperature while presents high density and low viscosity, which helps the system bomb. The Solutions that use H2O as refrigerant have a disadvantage, they cannot work in the evaporator at temperatures below 0°C, because water freezes at said temperature. Therefore, restricting its application only in AC in contrast with the ones that have NH3 as refrigerant, because they can work below 0°C and turn them apt to freezers.
ACKNOWLEDGEMENTS
The Author wants to thank to Consejo Nacional de Ciencia y Tecnología (CONACYT) and PIFI2014 for the support given for the work carried out for this work.
References
| [1] | M. Abdulateef, K. Sopian and M. A. Alghoul optimum design for solar absorption refrigeration systems and comparison of the performances using ammonia-water, ammonia-lithium nitrate and ammonia-sodium thiocyanate solutions, International Journal of Mechanical and Materials Engineering, Vol.3 19-23. (2008). |
| [2] | Wei Wu, Baolong Wang, Wenxing Shi, and Xianting Li Crystallization Analysis and Control of Ammonia-Based Air Source Absorption Heat Pump in Cold Regions, International Journal of Refrigeration 30 904-911,(2007). |
| [3] | I. Horuz, A comparison between ammonia-water and water-lithium bromide solutions in vapor absorption refrigeration systems, Int. Comm. Heat Mass Transfer, Vol. 25, No. 5, pp. 711-721, (1998). |
| [4] | Keith E. Herold, Reimhard Rader, acher, Stanford A, Klein, Absorption chillers and heat pumps, CRC Press P 53-193 (1996). |
| [5] | Dr Manuel R. Conde-Petrit Aqueous solution of lithium and calcium chlorides for use in air conditioning equipment design M., CONDE ENGINEERING, 2009. |
| [6] | Dr Manuel R. Conde-Petrit, Thermophysical properties of NH3+H2O solutions for insdustrial designe of absorption refrigeration equipment M. CONDE ENGINEERING, 2004. |
| [7] | Xiaohong Liaoa,* Reinhard Radermacherb, Absorption chiller crystallization control strategies for integrated cooling heating and power systems, Technologies Research Center, January 2007. |

















 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML