-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Condensed Matter Physics
p-ISSN: 2163-1115 e-ISSN: 2163-1123
2014; 4(3A): 8-18
doi:10.5923/s.ajcmp.201401.02
Optical Measurements of an Atmospheric Pressure Microplasma Jet Aiming Surface Treatment
J. C. Nascimento1, E. C. B. B. Aragão1, A. D. Fernandes1, F. T. F. Barbosa1, L. M. S. Costa1, D. C. Sousa1, C. Oliveira1, 2, G. J. P. Abreu1, K. G. Grigorov1, 3, P. Getsov3, R. S. Pessoa1, 4, V. W. Ribas1, B. N. Sismanoglu1
1Technological Institute of Aeronautics (ITA), Physics Department, São José dos Campos, Brazil
2Laboratório Nacional de Ciência e Tecnologia do Bioetanol–CTBE/CNPEM, Campinas, Brazil
3Space Research and Technology Institute, Acad. G. Bonchev Str. bl.1, Sofia, Bulgaria
4University of Paraiba Valley (Univap), Nanotecnology and Plasmas Processes Laboratory, Brazil
Correspondence to: B. N. Sismanoglu, Technological Institute of Aeronautics (ITA), Physics Department, São José dos Campos, Brazil.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
In this work stable non-thermal ac high voltage atmospheric pressure microplasma jet (APMJ) device was used for optical and electrical characterizations. It enables the generation of low power (~5W) microplasma jet at frequency of 60Hz. The jet has a visible radial diameter of approximately 1.5 mm. Optical emission spectroscopy was used as a diagnostic tool to determine the gas discharge parameters as the modes temperature. The rotational temperature of OH radicals at the exit nozzle varies from 325 to 525K for different gases where the electrical input power ranged from 3 to 10W. Both the electronic (0.5 – 0.7eV) and vibrational temperatures (0.35 – 0.58eV) were estimated at the same power conditions for Ar, He, N2, air and O2 + 1%Ar flow rates. The highly reactive species as OH, O, N2+ and the energetic photons produced between the electrodes extent along the plasma plume, both in radial and axial direction from the exit of the APMJ.
Keywords: Microplasma jet, Gas temperature, Optical emission spectroscopy
Cite this paper: J. C. Nascimento, E. C. B. B. Aragão, A. D. Fernandes, F. T. F. Barbosa, L. M. S. Costa, D. C. Sousa, C. Oliveira, G. J. P. Abreu, K. G. Grigorov, P. Getsov, R. S. Pessoa, V. W. Ribas, B. N. Sismanoglu, Optical Measurements of an Atmospheric Pressure Microplasma Jet Aiming Surface Treatment, American Journal of Condensed Matter Physics, Vol. 4 No. 3A, 2014, pp. 8-18. doi: 10.5923/s.ajcmp.201401.02.
1. Introduction
- The generation of atmospheric pressure microplasma jets (APMJ) using an alternating current (ac) power supply with high frequency and high rates of gas flow recently has been subject of extended studies. This growing interest is justified by the fact that the APMJ are suitable for numerous applications that requires low gas temperature at glow regime of operation with low input power. Among them, one can emphasize the surface treatment of heath sensitive bio-medical materials, thin film deposition, reactive ion etching, bio-medical and dentistry applications, etc. For bio-medical applications, the non-thermal APMJ must be at glow discharge regime possibly at room temperature. It is desired to maintain the discharge at Townsend-glow regime to produce parallel-operated jets but the related current is very low, resulting in low power plasma which finally results in formation of a low density active species. The latter are essential for bacteriological decontamination since the formation of OH radicals and O atomsis favored. Kim et al proposed a microplasma device which operates at 20kHz ac power using a commercial transform for a neon light coupled with a set of ballast resistor and autotransformer in order to avoid excessive currents [1]. A flow of 10L.min-1 nitrogen gas was injected through the microtube and ac voltage of 13.5kVp-p was applied to produce the jet, conditions suitable for use in biomedicine, as a cancer therapy. Szili et al used microplasma jet to modify surface at different treatment conditions [2]. To power the microjet at atmospheric pressure, a power delivery system consisting of a sinusoidal oscillator and an autotransformer (10kVp-p, 40mAp-p, 40kHz) was used. When polystyrene surface is plasma treated by this method, the contact angle measurements reveal increase of the surface energy transforming it as hydrophilic. Ayan et al developed an atmospheric pressure non-thermal microjet for localized surface treatment [3]. The applied power ranged from 0.1 to 0.2W, useful for polyethylene substrate treatment without causing any damage. The gas mixture consists of 0.2L.min-1 He + and 0.025L.min-1 O2 and surface functionalization (physical and chemical) was achieved. Seo et al designed a low-frequency (20 kHz, high voltage and mA current) He and Ar APMJ and measured its optical and electrical characteristics [4]. The high intensity of OH radical and O atoms in Ar plasma was suitable for large area sterilization and indirect treatment. Microplasma jet that operates at an excitation frequency of kilohertz ac range was developed to create nonthermal glow plasma. Here, He and O2APMJ were investigated by adding O2 flow, suitable for biomedical applications. He non-thermal microplasma jets generated at 50 kHz ac voltage were electrical and optically characterizes [5]. It has been detected a number of highly reactive species in the plasma plume such as OH, O, N2, He and N2+ at the glow discharge mode. The presence of numerous of Helines indicates higher plasma density and/or electron temperature. Great disadvantage of these microplasma jets is the high cost of both the He gas (generally L.min-1 He flow are needed to obtain a stable APMJ) and the equipment (high frequency power source used).The large surface to volume ratio of these miniaturized jets makes impossible surface treatments of a large scale. Furthermore, up to date there are not enough studies to detect and reducing the emission of a number of radicals such as HC, CO, NOx, O3 and SOx, harmful to health if inhaled and manipulated without a due care.Recently, an APMJ generated from micro-discharges in porous dielectric [6] or from the gap between two disk-shaped electrodes with a hole (1 mm of diameter) were presented [7]. Both used N2 and air to generate glow plasma powered by a high-voltage ac power supply and a commercially available transformer for neon light operated at 60Hz connected to the electrodes. These gases have higher dielectric breakdown voltages compared to He or Ar and in static mode (without gas flow) it is practically impossible to produce a glow discharge at atmospheric pressure. After the breakdown transition occurs from a glow discharge to a filamentary glow or silent electric discharge [8, 9] in a very short time due to this electrodes configuration. To avoid thermal instability leaving to a filamentary glow or glow-to-arc transition (transition from glow to thermal discharges), they generate the non-thermal and stable glow discharge through large gas flow rate (5 – 15L.min-1) that cools the plasma region removing the hot chemical species and neutrals. For surface treatment the presence of metastable states of some gases such as He (19.8 eV), Ar (11.6 eV) and N2 (6.2 eV) is very interesting to produce microplasma. These energetic species react with the surface chemical structure with large efficiency. In this work, a 60 Hz ac APMJ device was studied to produce stable cold plasma suitable for surface treatment. The electrical and optical characteristics will be raised for a range of input power and for these gases.
2. Experimental
- Figure 1 show the apparatus used to produce the ac microplasma jet. One transformer for neon light was used, operated at 60Hz. One can see in this figure the Cu electrodes, arranged parallel and inserted in a quartz tube. The nozzle cross-section area is about 5.3mm2 and the gap between the circular electrodes is about 1.2mm. The ac power supply (12kV, 40mA) is coupled with a variable autotransformer and with a ballast resistor (50kΩ, 100W) in order to avoid excessive current and to allow voltage control. This figure presents the setup used to perform optical emission spectroscopy (OES) measurements of APMJ. The plasma was confined between the electrodes, burning freely in air at atmospheric pressure (709Torr in the laboratory and 46% local humidity) and was composed by the main gas and, in some cases, high-purity 1% H2was added. The addition of H2 makes possible to estimate the electron number density of the plasma from the broadening of the hydrogen atom Hβ line. The gas connections were controlled by means of mass flow controllers MKS 247C and MKS model 1159B.The digital oscilloscope used in the experiments was a DPO7254 from Tektronix, 2.5 GHz. The highvoltageprobe (from Tektronix) was a P6015A and a current probe TCP 312 with amplifier TCPA 300. The monochromator used to capture the light from the APMJ was a IHR550 model from Horiba-Jobin- Yvon with adiffraction grating of 1800 linesmm-1 blazed at 500nm and 2400 linesmm-1 at 300nm. The entrance slit was fixed at 120µm and the respective instrumental Gaussian-type full-width at half-maximum (FWHM) ΔλI = 0.1nm was obtained by means of an Ar-Hg low-pressure scientific lamp and analyzing the fitting procedure for the Hg 435.8 nm strong line broadening. The spectra at the exit slit were recorded by a CCD Symphony with 1024×512 pixels and the SynerJy software was used to handle the spectra.
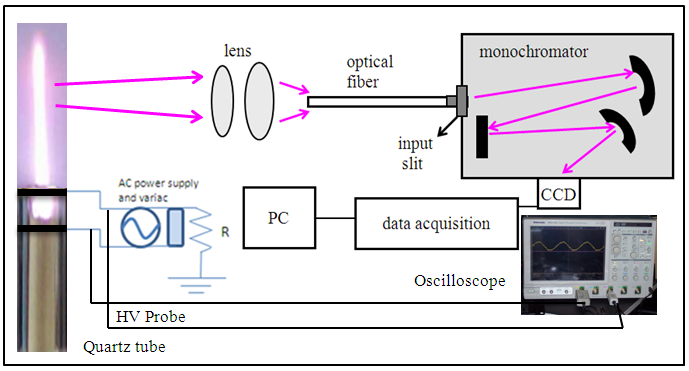 | Figure 1. Schematic representation of the microplasma jet and the schematic representation of the electrical circuit (the inset left represents the photograph of air plasma jet) |
3. Results and Discussion
- The frequency of the sinusoidal ac power supply is 60Hz and the glow discharge is achieved at a gas flow varying from 3 to 10 L.min-1. In Fig. 2 a typical current-voltage waveforms of Ar APMJ is presented, at low rate of about 6 L.min-1. The discharge voltage is about Vpp = 1200V (Vrms = 464 V) for current of Irms = 12.5mA. A filamentary discharge is observed at gas flow below 2L.min-1. The average electric power consumption was calculated by the equation
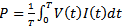 and results 4.8W.
and results 4.8W.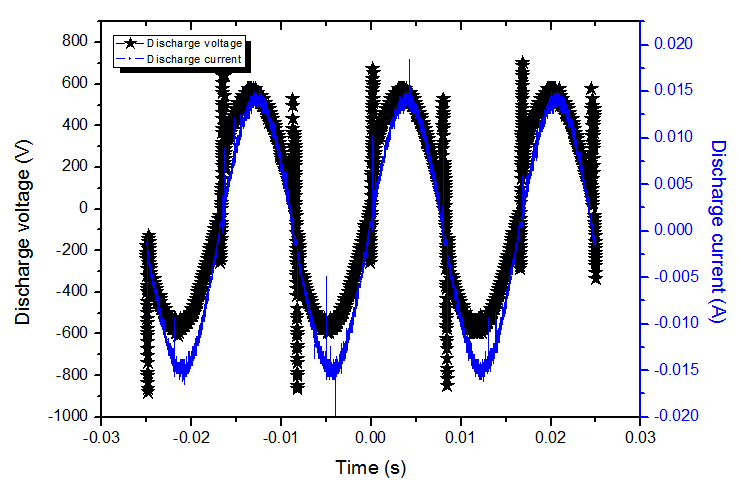 | Figure 2. Voltage and current waveforms of the Ar microplasma jet at Vrms = 464V and Irms = 10.4mA and flow rate of 6lmin-1 |
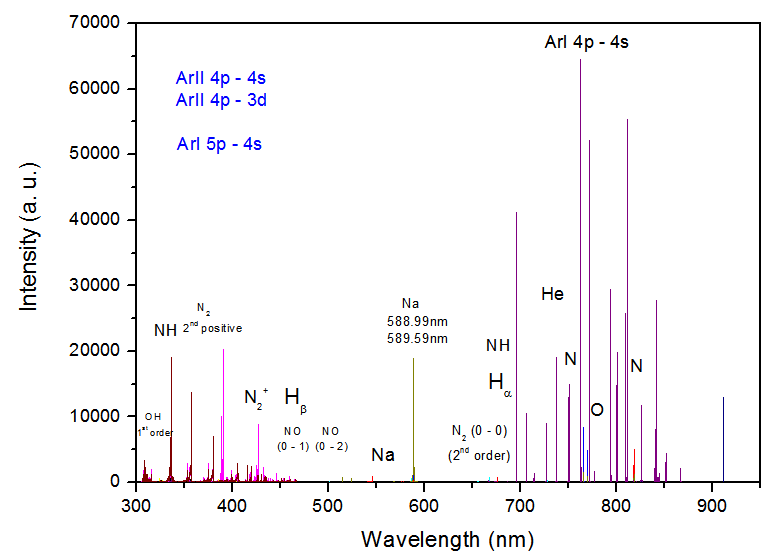 | Figure 3. OES measurements for a range of gas flow at APMJ |
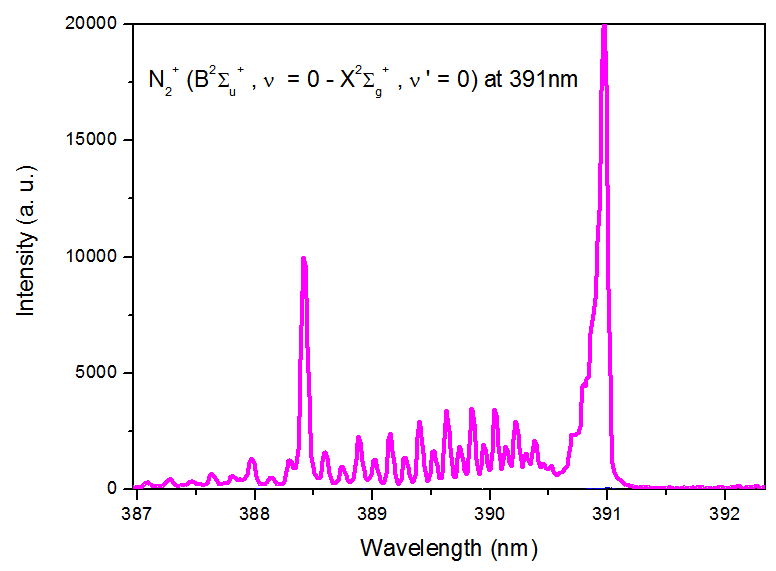 | Figure 4. N2+ at 391nm for He and Ar microjet in open air |
 When 2% of H2 is added with the He flow, both the Hβ line intensity and FWHM are increased. H atoms may be formed by reactions involving ionic He in H2 or in He. H+, H2+ and H3+ ions could be responsible for the observed Balmer atoms through the following reactions [11]
When 2% of H2 is added with the He flow, both the Hβ line intensity and FWHM are increased. H atoms may be formed by reactions involving ionic He in H2 or in He. H+, H2+ and H3+ ions could be responsible for the observed Balmer atoms through the following reactions [11]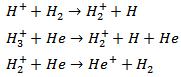 H2 may be dissociated by collision with electrons and may produce Hβ. The excitation of the H atoms should be done by electron impact
H2 may be dissociated by collision with electrons and may produce Hβ. The excitation of the H atoms should be done by electron impact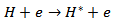 The spectra of OH bands were observed due to water vapor impurities in the gas and in the atmospheric air. The populations of excited state OH (A2Σ+, ν = 0 X2∏, ν’ = 0) at 306.4 nm can be generated by direct dissociation electron excitation of water or by dissociative recombination of H2O+, where this radical is formed by He metastables and subsequent dissociative recombination [12]
The spectra of OH bands were observed due to water vapor impurities in the gas and in the atmospheric air. The populations of excited state OH (A2Σ+, ν = 0 X2∏, ν’ = 0) at 306.4 nm can be generated by direct dissociation electron excitation of water or by dissociative recombination of H2O+, where this radical is formed by He metastables and subsequent dissociative recombination [12]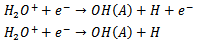 Figure 5 show the N2+ (B2Σu+, ν = 0 → X2Σg+ ,ν’ = 0; 380 to 475nm) for Ar and He flow (added with 2% oxygen gas) at the same power consumption, 4.8W. One can see the N2+ (B2Σu+, ν = 0 →X2Σg+, ν’ = 0) at 391 nm is practically suppressed when the plasma jet is operated with Ar + 2%O2, because this APMJ spend more power to produce oxygen atoms, reducing the production of vibrational species like N2+ [10]. The Ar I 603.2 nmline broadening (FWHM of the Lorentzian shape of the line, ΔλW(nm) = 3.9Tg0.7) is strongly gas temperature dependent and the weakness of this line intensity is an indication of the low gas temperature of the microplasma jet.
Figure 5 show the N2+ (B2Σu+, ν = 0 → X2Σg+ ,ν’ = 0; 380 to 475nm) for Ar and He flow (added with 2% oxygen gas) at the same power consumption, 4.8W. One can see the N2+ (B2Σu+, ν = 0 →X2Σg+, ν’ = 0) at 391 nm is practically suppressed when the plasma jet is operated with Ar + 2%O2, because this APMJ spend more power to produce oxygen atoms, reducing the production of vibrational species like N2+ [10]. The Ar I 603.2 nmline broadening (FWHM of the Lorentzian shape of the line, ΔλW(nm) = 3.9Tg0.7) is strongly gas temperature dependent and the weakness of this line intensity is an indication of the low gas temperature of the microplasma jet.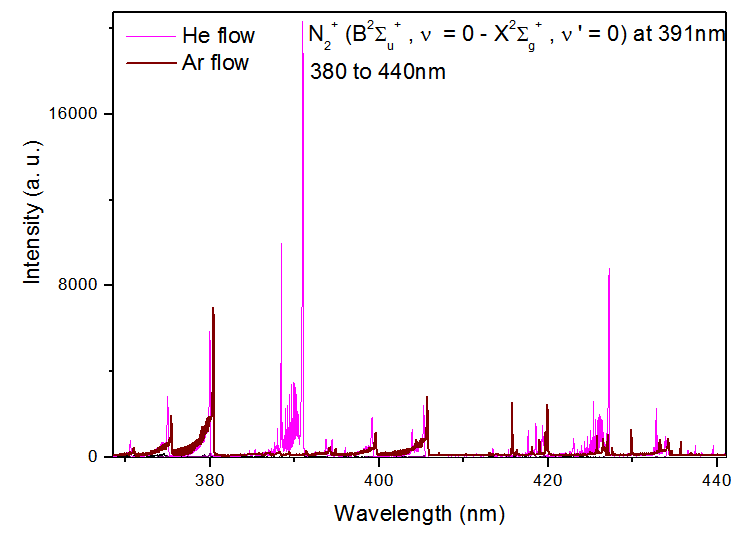 | Figure 5. N2+ (B2Σu+ ,ν = 0 → X2Σg+ , ν’ = 0; 380 to 440nm) for Ar and He flow at the same power consumption, 4.8W |
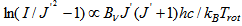 , where the rotational constant Bν = 1.815 cm-1.The linear fit of the Boltzmann plot gives the N2 rotational temperature. Figure 9 depicts the measured N2 rotational temperature obtained from Boltzmann plot at the same conditions of Fig.6, showing higher results for Trot by reasons explained before.
, where the rotational constant Bν = 1.815 cm-1.The linear fit of the Boltzmann plot gives the N2 rotational temperature. Figure 9 depicts the measured N2 rotational temperature obtained from Boltzmann plot at the same conditions of Fig.6, showing higher results for Trot by reasons explained before.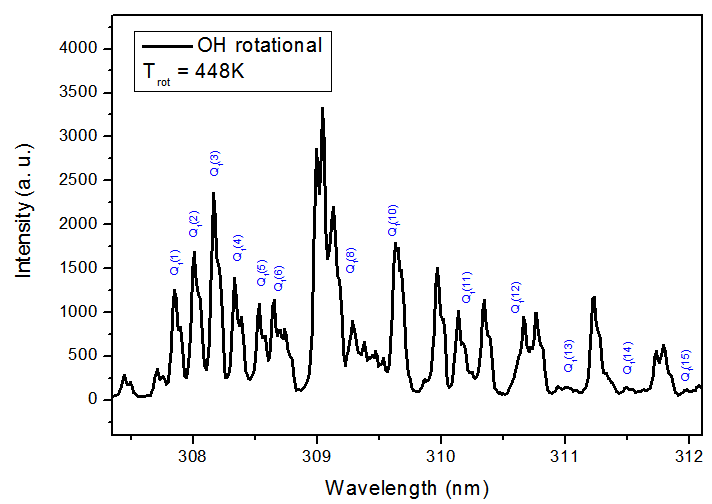 | Figure 6. UV emission bands of OH from 308 to 312 nm (Q1 branch) for Ar APMJ, Irms = 10.4 mA, Trot = (448 ± 33) K and flow of 6 L.min-1 |
 | Figure 8. Rotational spectrum of the N2 (C3∏u , ν = 0 → B3∏g, ν’ = 0) transition (R branch) |
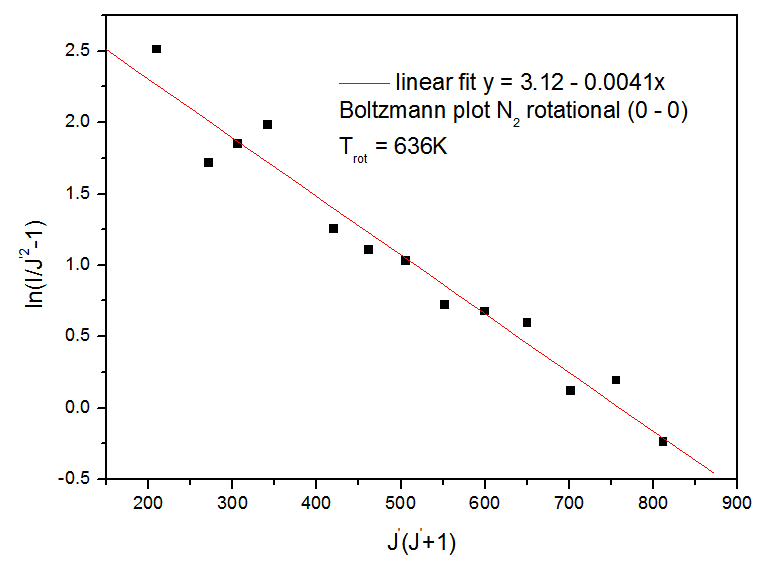 | Figure 9. Boltzmann Plot of the the N2 (0 – 0) (C3∏u ,ν = 0 → B3g, ν’ = 0) (R branch) rotational transitions (see Fig.8) |
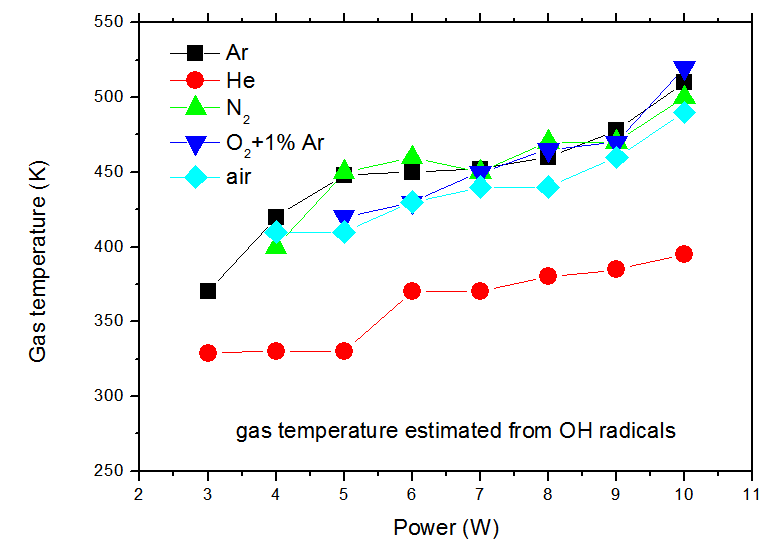 | Figure 10. Gas temperature as a function of the inputpower supplied to the discharge (6L.min-1 gas flow) |
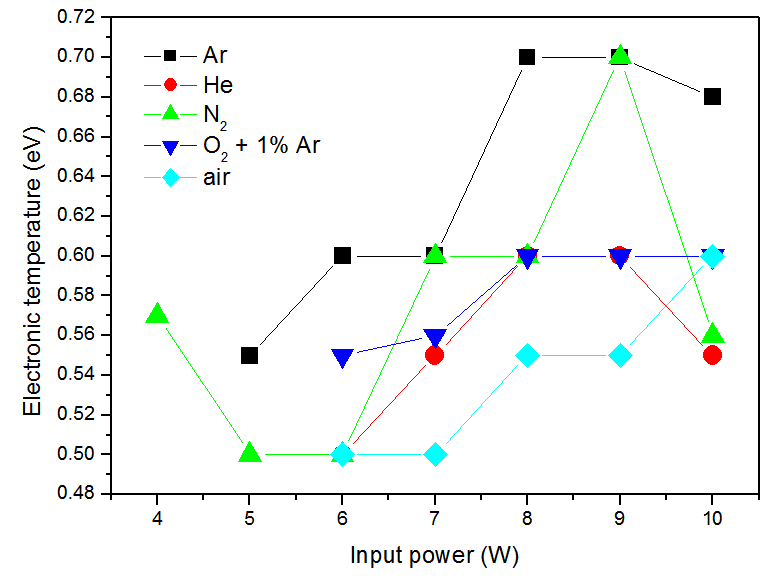 | Figure 11. Electronic temperature of the discharge versus the electrical input power |
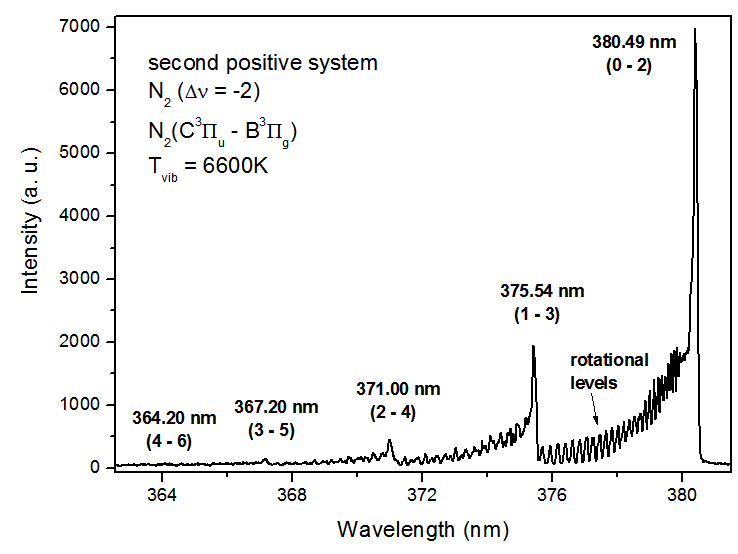 | Figure 12. Vibrational distribution of the N2 (C3∏u → B3∏g) |
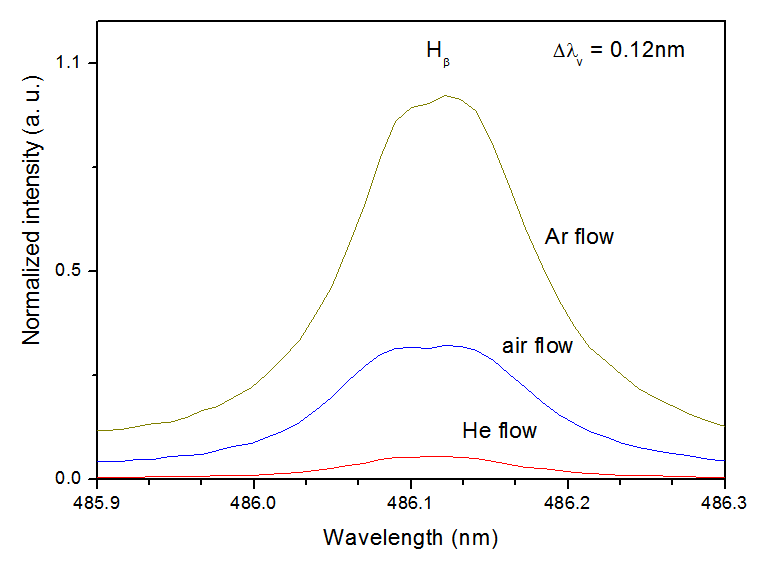 | Figure 13. Hβ line for Ar, air and He flow, at the same power consumption. |
4. Conclusions
- The non-thermal low frequency (60 Hz) ac atmospheric pressure microplasma jet (APMJ) could produce stable cold glow discharges using numerous of gases such as Ar, He, N2, O2 and air. The APMJ is suitable for surface treatment. The jet has a visible radial diameter of approximately 1.5 mm with afterglow surrounding the jet. Current and voltage measurements at flow rate of 6 L.min-1 show sinusoidal wave forms for flows of Ar, He, O2 + 1%Ar, N2 and air gases, with rms current and voltage amplitudes of: a) 464 V, 10.4 mA; b) 410 V, 10.6mA; c) 515 V, 13 mA; d) 430 V, 11.8 mA and e) 456 V, 12.9 mA respectively. The power absorbed by the plasma was: a) 4.8 W; b) 4.1 W; c) 5.8W; d) 4.4 W and e) 4.9 W, respectively for the mentioned species. The optical measurements show the emission of important lines for bio-medical and surface treatment applications, as O, N2, O3, N2+, N and OH. Electronic temperature (Te) was estimated through the Line Intensity Method. The average electronic temperature varies between 0.50 and 0.70 eV for a vast ranges of gas flow. The Boltzmann plot method was applied to estimate the vibrational temperature (Tv) in the plasma bulk using the second positive system N2 (C3∏u → B3∏g) for Δν = −2. The temperature was in the range 0.35 to 0.57 K for the input power varying from 3 to 10W, for all gases flow. The gas temperature Tg was estimated analyzing the OH (first order, ultra-violet, A2Σ+, ν = 0 X2∏, ν’ = 0) rotational band. A temperature decrease up to 40oC was observed up to 5 mm and beyond this range the gas temperature reaches the room temperature, enabling so the use of APMJ in treatment of heat-sensitive surfaces such as biomaterials and polymers. The difference between these three modes of temperature, Te>Tv>Tg, demonstrates near PLTE condition of this plasma source. The capacity of operation with molecular and atomic (noble) gases, including air, is important for various technological applications, but the novelty of this APMJ is the broadening of the plasma plume at the outer electrode with diameter of 2.5 mm and, consequently, reaching higher surface treatment area.
ACKNOWLEDGMENTS
- The authors acknowledge the financial support of the programs CAPES, FAPESP and CNPq for the partial financial support under Grant No. FAPESP/12/13064-4, FAPESP/PRONEX/11/50773-0, CNPq/MCTI/SECIS 406035/2013-0, CNPq/310419/2012-3 DT and CAPES/ 88881.030340/2013-01.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML