-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Advances in Analytical Chemistry
p-ISSN: 2163-2839 e-ISSN: 2163-2847
2015; 5(3A): 1-8
doi:10.5923/s.aac.201501.01
Immobilization of Acetylcholinesterase and Cholineoxidase on Mercapto-Carboxylic Acid Self-Assembled Monolayer on the Gold Electrode
Abdolhamid Hatefi-Mehrjardi1, Raziah Faiazi2
1Payame Noor University, Tehran, Iran
2Department of Chemistry & Nanoscience and Nanotechnology Research Lab (NNRL), Payame Noor University (PNU), Iran
Correspondence to: Abdolhamid Hatefi-Mehrjardi, Payame Noor University, Tehran, Iran.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
An electrochemical enzyme biosensor based on self-assembly on the polycrystalline gold electrode is constructed. The Acetylcholinesterase (AChE) and Cholineoxidase (ChO) are two enzymes that covalently co-immobilized on the mercaptopropionic acid self-assembled monolayer on the polycrystalline gold electrode (Au-MPA SAM). AChE catalyzes the hydrolysis of the neurotransmitter acetylcholine to acetate and choline that is oxidized by ChO and benzoquinone to yield the respective betaine and hydroquinone. The molecular dimension distances between two immobilized enzymes on Au-MPA SAM result in preconcentration of choline, in the ChO nano-environment. Hydroquinone, which is formed in the catalytic cycle, was measured amperometrically at a rotating disk electrode (RDE). The regeneration of benzoquinone from hydroquinone takes place at a fixed potential (0.35 V versus Ag/AgCl). The resulting current is a measure of the enzymatic activity. Carbaryl was chosen as a model toxin and AChE inhibition characteristics were utilized for the toxin detection. The biosensor fabrication steps are presented and discussed from which the biosensor is applied for carbaryl determination based on the Relative Inhibition (RI) of chronoamperometry responses by the addition of toxin.
Keywords: Electrochemical Biosensor, Carbaryl, Acetylcholinesterase Inhibition, Self-Assembled Monolayer, Electrochemical Impedance Spectroscopy, Chronoamperometry
Cite this paper: Abdolhamid Hatefi-Mehrjardi, Raziah Faiazi, Immobilization of Acetylcholinesterase and Cholineoxidase on Mercapto-Carboxylic Acid Self-Assembled Monolayer on the Gold Electrode, Advances in Analytical Chemistry, Vol. 5 No. 3A, 2015, pp. 1-8. doi: 10.5923/s.aac.201501.01.
Article Outline
1. Introduction
- With recent advances in nanotechnology, electrochemical biosensors, in combination with nanomaterials have become simple, efficient tools to measure the concentration of analytes and the response time functions of target biomolecules to the drugs or the toxic reagents [1]. Carbaryl (N-methyl-1-naphthylcarbamate; Sevin) is a carbamate nerve toxin that presents a potential teratogenic capability [2]. There are limited analytical methods available for the determination of carbaryl such as spectrophotometry [3], spectrofluorimetry [4], infrared spectroscopy [5], photothermal [6], chemiluminescence [7], quartz crystal microbalance [8], mass spectrometry [9] and chromatography. However, these techniques require expensive equipment as well as complicated and time-consuming solvent cleanup steps. Recently, some novel concepts to overcome these obstacles have been suggested. A promising alternative approach involves the use of enzyme-linked immunoassay (ELISA) [10]. While they have often demonstrated the required sensitivity and acceptable specificity, these assays need specific antibodies and, indirectly, the use of animals in order to produce these “receptors” [11].Great efforts have been devoted to the fabrication and characterization of a large variety of amperometric enzyme biosensors [12, 13]. The enzyme dissolved in electrolyte solution or immobilized on a solid electrode serves as a redox centre, and reacts selectively with biological species. The reaction product may be used directly or by mediation of a reversible redox couple for the determination of the species of interest. However, mediated electron transfer is the most efficient process and typically used for biosensors construction [14]. Immobilization of the enzyme on a solid electrode will decrease the distance between conducting substrate and enzyme redox centre, therefore, the electron transfer will be carried out near or inside the diffusion layer, which in turn, increases the sensitivity and selectivity of the biosensor [15]. The enzyme may be immobilized in a thin layer at the sensor surface in different ways; such as using polymers [16], carbon paste [17], monolayers [18], and multilayer self-assemblies [19], among them, immobilization via covalent attachment of enzyme to the functionalized self-assembled monolayers (SAMs) [20] is specially useful where miniaturization of the sensor in nanometre scales is required [21]. The functionalized SAMs formed on gold surface are ordered molecular assemblies, which are widely used for the immobilization of proteins and enzymes in biosensors fabrication [22].The biosensors usually contain two basic components connected in series: (i) A biochemical recognition system, which translates information from the biochemical domain into a chemical or physical output signal, and (ii) the transducer, which serves to transfer the sensor signal from the output domain of the recognition system to mostly the electrical domain. An electrochemical biosensor is a biosensor with an electrochemical transducer [23]. Most of the biosensor electrochemical transducers are based on potentiometric [24] or amperometric [25] detections. The amperometric detections are based on measurement of the current resulting from the electrochemical oxidation or reduction of an electroactive species, which is (i) a biocatalytic product (e.g. hydrogen peroxide), or (ii) a redox couple mediating redox enzymes and the electrode support at a constant potential. The earlier study [26] was shown that carbaryl inhibits the acetylcholinesterase (AChE), a key enzyme in the transmission of nerve impulses. On the basis of this effect, a method was developed to quantify carbaryl by measuring the decrease in enzyme activity, determined using modified Ellman's spectrophotometric method. The carbaryl inhibition effect on AChE extracted from different sources was studied and the implications in terms of kinetic mechanism and toxicity discussed [27, 28]. In this work, AChE and Cholineoxidase (ChO) are immobilized covalently on the topside of the gold Mercaptopropionic acid self-assembled monolayer (Au-MPA SAM) to produce Au-MPA-AChE/ChO SAMs as a working electrode (biosensor). Next, the biosensor is used to determine the carbaryl in the presence of parabenzoquinone (PBQ) mediator using chronoamperometry. The basis of the recognition system in this work is the diffusion of the carbaryl to the biosensor and inhibition of choline oxidation that resulted from acetylcholine hydrolysis. The data are presented and discussed from which a new method is proposed for carbaryl determination based on the chronoamperometry measurements.
2. Experimental
2.1. Chemicals
- Acetylcholine chloride and AChE from electric eel (500 U/mg, EC 3.1.1.7) ChO from Alcaligenes sp. (50 U/mg, EC 1.1.3.17), carbaryl, pralidoxime iodide (2-PAM), 3-mercaptopropionic acid (MPA), Bovine serum albumin (BSA), N-hydroxysuccinimide (NHS), parabenzoquinone (PBQ), 1-ethyl-3-(3-dimethyl aminopropyl)carbodiimide hydrochloride (EDC), and other chemicals were of commercial sources (Merck or Sigma) and used as supplied without further purification except parabenzoquinone that was recrystallized from hot solution of n-hexane. All solutions were prepared with double-distilled water. Phosphate buffer solutions (PBS) contained 0.05 M KCl, 0.05 M K2HPO4/KH2PO4 were used and the pH was adjusted with NaOH or H3PO4 dilute solutions. The biosensor was then kept in dry condition at 4°C until used.
2.2. Electrode Modification
- The polycrystalline gold working electrode (0.0314 cm2, Azar electrode Co. Urmia, IRAN) was polished using aqueous slurries of alumina (0.3 to 0.05 µm), sonicated in three steps: in water, chloroform and water each for 5 min, and then cleaned electrochemically by cycling the electrode potential between 0.000 and +1.500 V vs. Ag/AgCl in 0.5 M sulfuric acid until reproducible voltammograms were observed [29]. A roughness factor of 1.8 ± 0.1 was obtained from ratio of the real to geometric surface area of the electrode [30], and attempted to maintain it constant in all experiments. The cyclic voltammograms obtained on the electrode in the presence of reversible marker, Fe(CN)63-, showed a peak separation that confirms the safety of the system (ΔEp ≅ 60 mV). Immediately before modification, the electrode was thoroughly rinsed with distilled water. Cleaned gold electrode was modified by placing into a 25:75 (v/v) water/ethanol solution containing 70 mM MPA for 12 h to form Au-MPA SAM electrode. The modified electrode was washed with the same ethanolic solution, dried in argon stream, and activated in PBS (pH = 5.5) containing 0.002 M EDC and 0.005 M NHS for 2 h. Then, the electrode was rinsed with the same PBS and immediately an aliquot of 50 µl of 2 mg/ml AChE and 100 mg/ml ChO enzymes in PBS (pH 7) was dropped onto it for at least 1.5 h to fabricate Au-MPA-AChE/ChO SAMs electrode, washed with PBS, and used for electrochemical measurements.
2.3. Electrochemical Measurements
- A conventional three-electrode cell, consisting of Au-MPA-AChE/ChO SAMs modified electrode as working, Ag/AgCl, KCl (sat’d) as reference, and a platinum foil with large surface area as auxiliary electrode, was used for electrochemical measurements. The measurements were carried out using Potentiostat/Galvanostat Autolab 12 equipped with Frequency Response Analyzer (FRA 4.9), interfaced with a personal computer, and controlled by GPES 4.9 and FRA 4.9 softwares (Eco Chemie BV, Utrecht, The Netherlands).The electrochemical characterization of Au-MPA SAMs electrode was performed in the presence of 0.5 mM K3Fe(CN)6 redox probe using EIS and CV. Quantitative determination of carbaryl was performed in the presence of 5 mM PBQ as a mediator by chronoamperometry. For the characterization of the modified electrode, the DC potential was formal potential of the redox couple, (i.e. E0΄= 0.200 V vs. Ag/AgCl for [Fe(CN)6]3-/4- and E0΄= 0.080 V vs. Ag/AgCl for PBQ/H2Q). Quantitative determination of carbaryl was performed in different DC potentials. Other experimental conditions are described in the respective figures.
2.4. Analytical Procedure
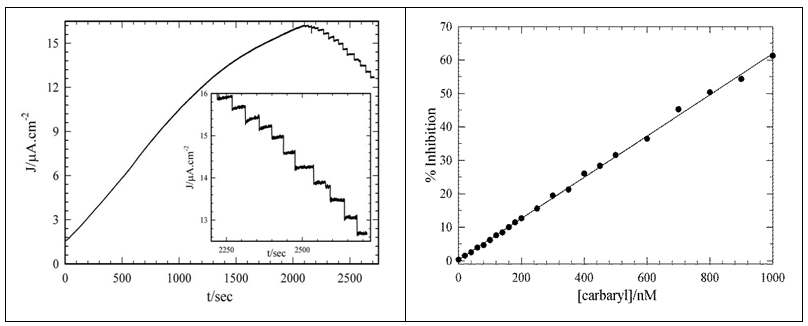
- The Au-MPA- AChE/ChO SAMs modified electrode was allowed to settle to a stable current over a period of at least 0.5 h in the background solution. Background solution was the 10 ml of PBS (pH 7.0) containing 2.0 mmol l−1 acetylcholine as the enzyme substrate and 5 mM PBQ as the electron mediator. Prior to each experiment, the solutions were bubbled with high-purity argon gas for at least 20 min and blanketed with the same gas during the experiments to eliminate oxygen interference. All experiments were carried out at room temperature. Once the electrode background current was stable, additions of carbaryl were made from the stock solution using micropipette. The degree of inhibition, in percentage, was calculated as the relative decay of the biosensor response after the contact with the carbaryl.
3. Results and Discussion
3.1. Fabrication and Characterization of Au-MPA SAMs Electrode
3.1.1. Formation of SAMs
- For enzyme electrode a short alkyl chain alkanethiol, usually three carbons long, is used so that the grafted redox centre (e.g. an enzyme) being as close as possible to the electrode. An additional advantage of the short alkyl chain is that a relatively disordered SAM is formed which means the underlying metal is still electrochemically accessible. In this study MPA was selected as a short alkyl chain to bind AChE/ChO into the Au electrode surface, and form Au-MPA-AChE/ChO SAMs biosensor. The schematic diagram of biosensor preparation is shown in Fig. 1. The formation of SAMs was traced by CV and EIS.
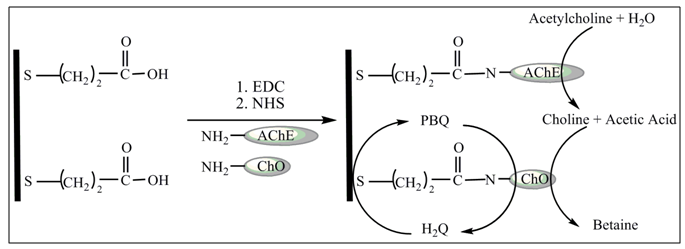 | Figure 1. Schematic illustration of the immobilization of AChE and ChO on gold electrode using a SAM of MPA and EDC-NHS as coupling agents |
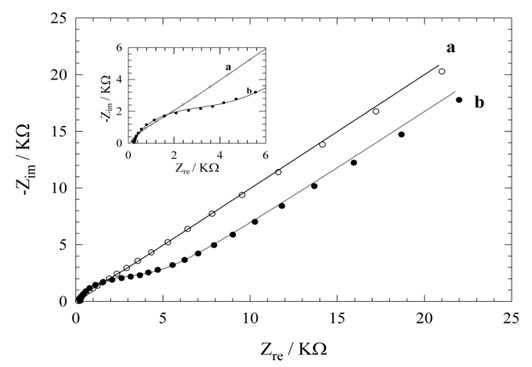
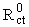 = 1114 Ω from the high frequency range semicircle) followed by straight line indicating domination of mass diffusion limiting effect on the electron transfer process (curve a, in the low frequency range). The respective semicircle diameter at the high frequencies range, corresponding to the charge transfer resistance at the electrode surface, increases upon the MPA SAMs formation on the gold electrode surface (curve b, Rct = 3730 Ω).The formation of MPA SAMs on the electrode surface has produced a larger barrier to the interfacial charge transfer, which is revealed by the increased diameter of the semicircle in the spectrum. However, the charge transfer resistance is not infinitive and there is still diffusion line (Warburg impedance). This means that the surface is not completely blocked and the charges can penetrate to the gold surface producing faradaic current. Since the EIS measurements are made at formal potential where no overpotential acting on the electrodes, the EIS results particularly Rct, indicate the information of surface coverage [31].
= 1114 Ω from the high frequency range semicircle) followed by straight line indicating domination of mass diffusion limiting effect on the electron transfer process (curve a, in the low frequency range). The respective semicircle diameter at the high frequencies range, corresponding to the charge transfer resistance at the electrode surface, increases upon the MPA SAMs formation on the gold electrode surface (curve b, Rct = 3730 Ω).The formation of MPA SAMs on the electrode surface has produced a larger barrier to the interfacial charge transfer, which is revealed by the increased diameter of the semicircle in the spectrum. However, the charge transfer resistance is not infinitive and there is still diffusion line (Warburg impedance). This means that the surface is not completely blocked and the charges can penetrate to the gold surface producing faradaic current. Since the EIS measurements are made at formal potential where no overpotential acting on the electrodes, the EIS results particularly Rct, indicate the information of surface coverage [31].3.1.2. Determination of Surface Coverage of Au-MPA SAMs
- The Total surface coverage, Г of the MPA SAMs on gold, may be evaluated by integrating the cathodic peak associated with desorption process obtained in NaOH solution by CV [32]. The charges consumed for the MPA desorption, measured from the first cyclic voltammograms (corrected for background) was 53 μC/cm2. Assuming one-electron reduction process (Au-SR + e- → Au + RS-) the charge of 53 μC/cm2 was further converted to a surface concentration of 5.55 × 10-10 mol/cm2. Comparing this value with ideal value of 7.68 × 10-10 mol/cm2 for the packing arrangement close to (√3 × √3) R30 [33] shows that almost 73 ± 7% of the gold electrode surface is covered by MPA. It should be emphasized that Au-MPA SAMs is stable in the applied potential range between +0.800 and -0.700 V vs. Ag/AgCl, which is a suitable potential window to study most of biological redox species. The partial surface coverage (θ) was also estimated using
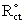 = 1114 Ω and Rct = 3730 Ω for uncovered and covered gold electrodes (Fig. 2, curve a and b), and the relation θ = (1 -
= 1114 Ω and Rct = 3730 Ω for uncovered and covered gold electrodes (Fig. 2, curve a and b), and the relation θ = (1 - 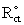
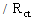 ) [34], as 70 ± 5%. This value is in good agreement with the value obtained by CV. However, it should be mentioned that it is difficult to find a perfect baseline in CV plots.
) [34], as 70 ± 5%. This value is in good agreement with the value obtained by CV. However, it should be mentioned that it is difficult to find a perfect baseline in CV plots.3.2. Enzymes Immobilization
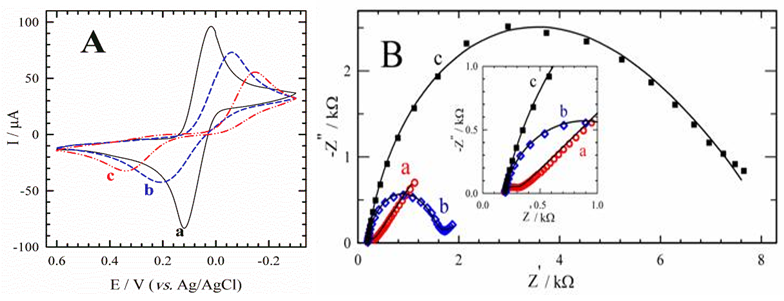
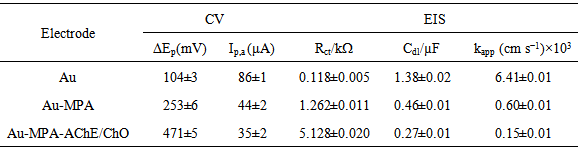
- The two enzymes AChE and ChO can be co-immobilized onto the surface of the working Au electrode in the same or separate immobilization matrices or, one can be immobilized and the other can be added in solution. The co-immobilization of the two enzymes generally allowed detection of analytes in a lower concentration range [35]. To bind enzyme molecules tightly onto the Au electrode surface, the amine groups of the AChE and ChO were coupled to the acidic head groups of Au-MPA SAMs through the formation of imide groups using EDC and NHS, according to the literature [36]. Therefore, the immobilized enzymes on the gold surface and the solubilized carbaryl in the electrolyte solution could react near the surface of the gold electrode. AChE catalyzes the hydrolysis of the neurotransmitter acetylcholine to acetate and choline that is oxidized by ChO to yield the respective betaine. However, when choline reduces the ChO enzyme-bound FAD, FADH2 forms, which cannot directly go to the electrode surface and oxidize, because of fixation of FAD to the enzyme molecule. To solve this problem an organic electron mediator such as parabenzoquinone (PBQ) was chosen as a mediating agent between ChO and electrode. Cyclic voltammetry is a simple and easy tool to show the changes of electrode behavior after each assembly step, because the electron transfer between the solution species and the electrode must occur by tunneling through either the barrier or the defects in the barrier. Fig. 3A shows the cyclic voltammograms obtained on the bare Au (curve a), Au-MPA SAMs (curve b), and Au-MPA-AChE/ChO SAMs (curve c) electrodes in the presence of 5 mM PBQ redox mediator. While the redox probe exhibits quasi-reversible electrochemical features at the bare Au electrode, immobilization of the MPA SAMs onto the electrode results in a peak separation. Upon the assembly of the enzymes on the Au-MPA SAMs, the interfacial charge transfer between the redox probe and the electrode is further blocked and maximum peak separation occurs. Quantitative parameters extracted from CV data are summarized in Table 1. Also, according to EIS data (Fig. 3B), the complex plane plots obtained on the clean bare Au, Au-MPA and Au-MPA-AChE/ChO SAMs electrodes at the same solution conditions. The bare gold electrode shows a very small semicircle (curve a, from the high frequency range semicircles) followed by straight line indicating domination of the mass diffusion limiting effect on the electron transfer process (curve a, in low frequency range). The layer by layer assembly of thiol (curve b) and enzymes (curve c) on the gold electrode impedes the electrode surface and produce a larger barrier to the interfacial charge transfer, which is reveal by the increased diameter of the semicircle (Rct) in the spectra and decreased apparent rate constant (kapp) (Table 1).
|
3.3. Chronoamperometric Response
- Reversible inhibitors can bind to enzymes through weak non-covalent interactions such as ionic bonds, hydrophobic interactions, and hydrogen bonds. Because reversible inhibitors do not form any chemical bonds or reactions with the enzyme, they bind rapidly and can be easily removed; thus the enzyme and inhibitor complex is rapidly dissociated in contrast to irreversible inhibition. Carbaryl is known to involve in the reversible inhibition on AChE [37]. Therefore, the immobilized AChE enzyme activities diminish to its substrate, acetylcholine in the presence of carbaryl. Due to carbamylation of the serine residue at the active site of the AChE enzyme by carbaryl, the production of the choline from acetylcholine decreases and so diminishes the oxidation current related to enzymatic reaction product through the ChO enzyme and mediator which in turn PBQ is oxidized at Au electrode surface in diffusion layer. A simple hydrodynamic chronoamperometry method for the determination of carbaryl was established due to remarkable current decreasing in the amperometric signals of the Au-MPA-AChE/ChO SAM electrode by successive carbaryl addition to the cell solution containing acetylcholine substrate and PBQ redox mediator (Fig. 4).
4. Conclusions
- We first studied the formation of a surface with carboxylic acid functional groups that in molecular dimension close to the electrode surface. Then the surface carboxylic acid functional groups are activated by some activator agents to link with amine groups of a few enzyme amino acids to form amide bonds and by the way the enzymes were immobilized covalently, close to the gold surface. The resulting AChE/ChO biosensor in the presence of acetylcholine substrate and appropriate redox mediator (PBQ) exhibited high sensitivity, good reproducibility, long-term stability and low-cost processes, which provided a new promising tool for the characterization of enzyme inhibitors and pesticide analysis. These results suggest that the fabricated bienzymatic self-assembled monolayer biosensor can be successfully used for the electrochemical determination of carbaryl in real samples.
ACKNOWLEDGEMENTS
- The authors gratefully acknowledge the Sirjan Payame Noor University providing research facilities for this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML