-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
p-ISSN: 2325-002X e-ISSN: 2325-0038
2019; 9(1): 7-11
doi:10.5923/j.zoology.20190901.02

Density and Feeding Related Mortality of Musca domestica (Lin.) Larvae (Diptera: Muscidae)
Yahaya M. M.
Department of Biological Sciences, Usmanu Danfodiyo University, Sokoto, Nigeria
Correspondence to: Yahaya M. M., Department of Biological Sciences, Usmanu Danfodiyo University, Sokoto, Nigeria.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Effects of varying densities of larvae and feed with an amino acid lysine (1.5g) additive on the mortality of larvae and growth of pupae of Musca domestica (L.) were determined. Larvae were initially kept at density of 3 larvae per group in a beaker and fed with 40 per cent milk (w/v) concentration. Both the density and feeding concentrations were increased progressively. Mortality of the larvae was zero at 3 larvae per group but the mean axial ratio of puparia was observed to be 13.75±0.33. As the number of larvae increased subsequently from 3 to 6, 12, 24, 42 and 74 per each group, varying mortality values and marked reduction in axial ratios of the puparia were observed. Increasing mortality rate was also observed with the constant number of larvae kept at 24 per group at different feeding regimes in some cases. Lysine additive however, seemed to influence the reduction of mortality rate but only slightly improved mean axial ratios of puparia. This suggested that factors other than food also affected survival of larvae and growth of pupae in crowding conditions.
Keywords: Crowding, Feeding regimes, Larvae, Mortality, Musca domestica
Cite this paper: Yahaya M. M., Density and Feeding Related Mortality of Musca domestica (Lin.) Larvae (Diptera: Muscidae), Research in Zoology , Vol. 9 No. 1, 2019, pp. 7-11. doi: 10.5923/j.zoology.20190901.02.
Article Outline
1. Introduction
- Musca domestica (L.) commonly known as housefly is widely distributed and familiar to many as domestic pest, nuisance fly and vector of variety of pathogenic organisms. Its serious insect not only for their annoyance factor but also for being major vectors of several disease- inducing agents [1]. Similarly, its habit of visiting garbage, excrement and human food all in one short flight makes it capable of transferring organisms that cause diarrhea, dysentery, typhoid, cholera and other diseases to human [2]. Expanding of the fly food range to include human garbage and moving indoors makes it to become a cosmopolitan pest and found wherever humans have settled [3,4]. M. domestica represents pest of major economic significance and pollution of animal products, poultry, livestock and the transfer of the wide range of animal pathogens [5]. The continued success of M. domestica in different parts of the world can be explained by at least two important factors, a high growth rate and short life cycle [6]. In fact, housefly represents the ultimate adaptation to the human environment by an insect [7].The fly is a holometabolous with distinct egg, larva or maggot, pupa and adult stages. The whitish eggs each measured 1-2mm in length are laid singly and directly on acceptable substrate but pile up in small masses [8]. A good breeding medium is animal or poultry manure [9]. Up to 500 eggs can be laid per female in several batches of 75-150 over a three to four day period [10]. Number of eggs produced however, is a function of female size, which is principally a result of larval nutrition [11,12]. Normally the egg laying females are attracted to warm and moist (40-70% water) organic substrates that may provide adequate nourishment for the hatched larvae [7]. Maximum egg production occurs at intermediate temperature, 25-30°C [13,14]. The first instar larva (3-9mm long), a typical creamy whitish in colour, cylindrical but tapering toward the head [15,16], hatched in about 8 hours or up to 3 days depending on temperature [7,13]. Larvae burrow into the food material to feed, but complete the three instars (3rd instar larvae, 7-12mm long) and pupate in a week or less [17,18]. The optimum temperature for larval development is 35 to 38°C, though larval survival rate is highest at 17-32°C [19,20]. Pupa is dark-brown 8mm long and under warm conditions last 4-6 days [7,16]. The emerging adult fly, 6-9mm in length [7] escapes from the pupa case through the use of an alternately swelling and shrinking sac called the ptilinum on the front of its head, which it uses like pneumatic hammer to break through the case [8,12] with the female usually larger than the male [16]. Warm summer conditions are generally optimum for the development of the housefly and it can complete its life cycle in as little as seven days [14]. As many as 10-12 generations may occur annually in temperate regions, while more than 20 generations may occur in subtropical and tropical regions [21,22]. Previous work done on housefly have indicated that larval crowding affect pre-imaginal mortality [23], adult size proportional to life span [24] and that several factors like size and aggressiveness determined success in competition for food [25].
2. Objectives
- This paper determines the effect of three variables; larval crowding, feeding regimes and lysine additive on the mortality of housefly larvae and size of the puparia.
3. Materials and Methods
3.1. Collection of the Flies
- Adult houseflies, M. domestica were collected from insect swarm in Sokoto main abattoir using insect sweep net. The technique used was by throwing the net at angle of 45° to the horizontal and then rose to about 90°; in this a lot of the flies were caught. This was immediately followed by squeezing the top side of the net with hand to disallow any fly escape. The adults were brought to the laboratory where both sexes were sorted and identified through taxonomic procedure [26].
3.2. Breeding Procedure
- For the rearing purpose, adult flies were transferred into a population cage sized 30 x 30 x 30cm covered with mosquito net, having one side provided with a sleeve to allow for easy access to the inside through which food container could pass in or out of the cage. Floor of the cage was sprayed with green manure to enhanced oviposition. The flies in the cage were fed with 40% (w/v) of NIDO milk solution soaked in 30g of cotton wool, since the adults would require food (protein) before copulation can take place [22]. Eggs laid by the female flies in Petri dishes and on cotton wool already placed in the cage were collected and placed in a beaker (100mL capacity) containing three pieces of 20g cotton wool moist with 40% (w/v) of NIDO milk solution. The beaker was covered with muslin cloth to keep away other insects from entering. After every 24hrs, the cotton wool was re-moist again with the same milk solution. Hatched larvae obtained in the beaker were allowed to pupate and the resulting pupae were placed into a similar population cage. Within three weeks of this procedure, large number of adults was obtained. The purpose was to procure a third generation flies from which eggs were removed and reared for the experiment.
3.3. Experiment Conducted
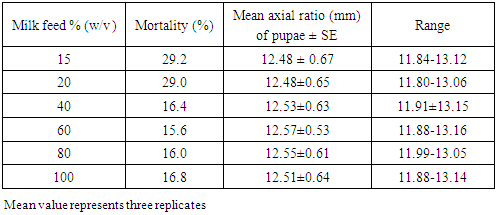
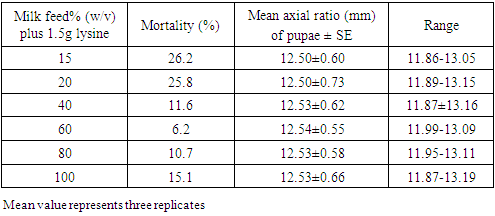
- From the third generation flies maintained as stock, variable number of individuals per group of larvae (3, 6, 12, 24, 42 and 74) was sampled using camel hair brush and then placed in separate but similar beakers containing cotton wool soaked in 40% (w/v) milk solution to serve as larval food. Six concentrations of the milk were prepared (15, 20, 40, 60, 80 and 100% w/v) and from each also, cotton wool was soaked and placed in 100mL beaker to feed 24 larvae within. In another set up, while maintaining different concentrations of milk feed and density of larvae at 24 per group, additional cotton wool soaked in 1.5g amino acid lysine solution was also placed to feed the larvae on each concentration. Observations were made daily for larval mortality and all the larvae considered dead were removed from the beaker and their number was counted. The axial ratio of puparia (size of the pupa) was measured in millimeters with venire calliper (Mutoyo: Japan) upon pupation before eclusion. All experiments were replicated three times.
3.4. Data Analysis
- Larval mortality was expressed in per cent as the difference in the number of dead larvae and pupae to the initial number of larvae introduced [27]. Analysis of Variance was used to calculate the mean size of pupae and expressed as the mean axial ratio.
4. Results and Discussion
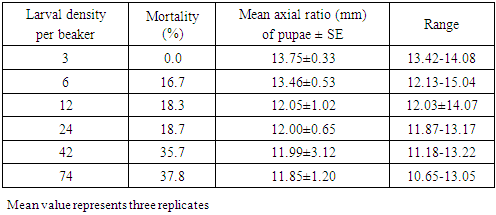
- Crowding effect on the mortality of M. domestica larvae is presented in Table 1. There was no larval mortality observed in less crowding condition of three larvae per beaker, while the mean axial ratio of the resulting pupae was observed to be 13.75±0.33. When the density of larvae was increased twice, a record of 16.7% mortality was obtained indicating that the effect of crowding was slowly building but the mean axial ratio of pupae had only changed slightly (13.46±0.53) from the previously observed mean when larval crowding was as least. This observation could not be rationally attributed to inadequate space or food since both were available in this case. However, some authors have proposed possible factors for relatively high mortality at very low densities in tadpoles of Rana pipiens as polluting effect of food excess caused by increase in dissolved oxygen or increase in toxins [28]. It was confirmed that during larval stage of M. domestica there was elimination of ammonia which is toxic and also oxygen consumption decreases while carbon dioxide production increases [29]. Larval mortality steadily increased with every rise in crowding, so also the reduction in the pupal mean axial ratio with the highest reduction (11.85±1.20) observed in highest larval density (74 per beaker). Similar observations were made previously when working with Drosophila melanogaster [15, 25] and with M. domestica [30].
|
|
|
5. Conclusions
- This study has shown that, crowding of M. domestica larvae was indirectly proportional to larval mortality. That the larvae have shown a particular preference for milk feed concentration but the amino acid lysine additive in feed could not achieve marked increase in the axial ratio of puparia. Some other factors not exploited presently might also be responsible for high larval mortality.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML