-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
p-ISSN: 2325-002X e-ISSN: 2325-0038
2017; 7(1): 1-6
doi:10.5923/j.zoology.20170701.01

Geometric Morphometric Comparison of Namak Chub (Squalius namak, Khaefi et al., 2016) in Rivers of Lake Namak Basin of Iran
Atta Mouludi Saleh, Yazdan Keivany, Seyed Amir Hossein Jalali
Department of Natural Resources (Fisheries Division), Isfahan University of Technology, Isfahan, Iran
Correspondence to: Yazdan Keivany, Department of Natural Resources (Fisheries Division), Isfahan University of Technology, Isfahan, Iran.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Namak chub (Squalius namak Khaefi et al., 2016) is an endemic fish species in the inland waters of Namak Lake basin in central Iran. To investigate the patterns and the form changes using geometric morphometric methods, 77 specimens were collected from three rivers of the Namak basin. After anesthetizing in 1% clove oil solution and fixing in 10% neutralized formalin, the specimens were transferred to the Isfahan University of Technology Ichthyology Museum (IUT-IM) for further studies. Some 13 landmarks were made on the photographs taken from the left side of the fish to extract data from body for geometric morphometric analysis using digitization TpsDig2 software. Defined data, after Procrustes Analysis, were analysed by principal component analysis, canonical variate analysis and cluster analysis. The results showed that there were significant differences among the studied populations (P<0.0001). Much of these differences are related to the head, mouth position, body depth, anal fin position and width of the caudal fin. This indicates that the there is a polymorphism associated with populations habitat conditions. The streamlined body shape was seen among populations as a common feature that for chub species was considered as an advantage in rivers.
Keywords: Morphometric, Landmark, Procrustes, Morphological patterns
Cite this paper: Atta Mouludi Saleh, Yazdan Keivany, Seyed Amir Hossein Jalali, Geometric Morphometric Comparison of Namak Chub (Squalius namak, Khaefi et al., 2016) in Rivers of Lake Namak Basin of Iran, Research in Zoology , Vol. 7 No. 1, 2017, pp. 1-6. doi: 10.5923/j.zoology.20170701.01.
Article Outline
1. Introduction
- Fishes are the most diverse and abundant vertebrates and are distributed across the world waters and this is due to the amazing diversity in their behavior, physiology and morphology [1-3]. Morphological characters including meristic and morphometric characters aimed at identifying populations has a long history in biology [4]. Studying flexibility of morphology among individuals of the same species could facilitate the understanding of environmental effects on different populations [5]. Fish can react in a relatively short time to the environmental conditions which they live in and create different populations with different morphological patterns [6]. Two types of morphometric techniques are used, traditional based on statistical analysis of measured distances, such as length, width and depth of the body and geometric, based on collecting data such as the curved trajectory of peripheral landmarks and semi-landmarks, on biological structures. Difference genetic and morphological plasticity and environmental factors can created morphological differences among different populations of a species. Environmental factors influencing body shape in particular species in different habitats factors can exert themselves through natural selection causing isolation of populations of species in different habitats. [7, 8]. Biological factors affecting the process of evolution, such as competition, predation, the availability of food resources and the physical parameters such as the type of substrate, water depth, vegetation, the effects of human manipulation, such as dams, modify and change traits including body shape, eating and swimming patterns and reproductive behavior and the overall changes are influenced by the interaction of these factors [9, 10]. Based on the specific characteristics of each region, it differently increases the efficiency of a selected shape. So it is possible that morphological characteristics be advantageous in a habitat, but disadvantageous in other habitats [11]. Chubs of the genus Squalius are widespread in Europe and the Middle East. The genus has a high species diversity, especially in the Mediterranean, Caspian and Urmia basins [12]. Hence, the present study was conducted to evaluate the geometric morphometric of chub populations in different rivers.
2. Materials and Methods
2.1. Sampling
- Lake Namak (salt) basin (51°52'0.00"E, 34°30'0.00"N) in central desert of Iran, is located between Isfahan, Qom and Semnan provinces. For this study, a total of 77 specimens of S. namak were collected using seine nets from three rivers of Namak basin in 2010- 2011. (Ghinercheh: 24 (49°12'2.00"E, 34°30'15.00"N), Qomrud; 29 (50°32'12.00"E, 34°18'50.00"N) and Jajrud: 24 (51°42'25.08"E, 35°40'44.62"N) (Fig. 1). After anesthetizing the specimens in 1% clove oil solution and fixing in 10% neutralized formalin, they were transferred to the Isfahan University of Technology Ichthyology Museum (IUT-IM) for further studies. The voucher number of samples has been recorded as IUT-IM13880602-142-01, IUT-IM13880518-089-02 and IUT13890316-025-03.
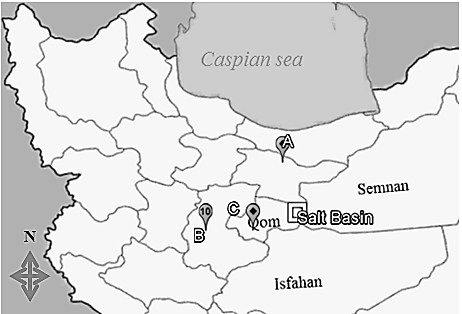 | Figure 1. The sampling locality of Squalius namak populations in Namak lake Basin (A: Jajrud, B: Ghinercheh, C: Qomrud) in Iran |
2.2. Laboratory Work and Data Analysis
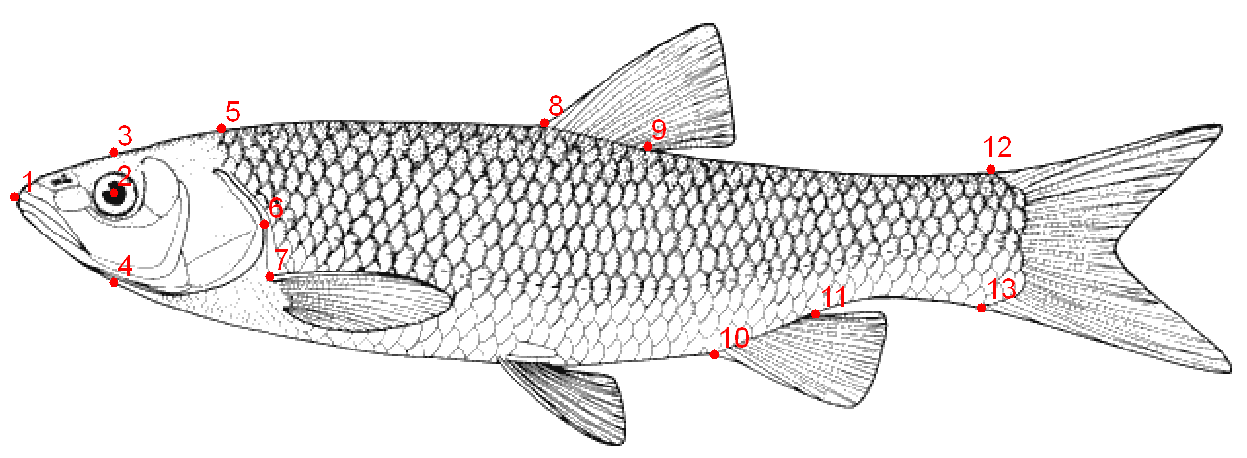
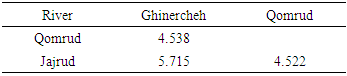
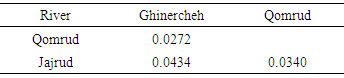
- The left sides of the specimens were photographed using a Canon digital camera (8 MP). Some 13 landmarks on two-dimensional images were selected using Tpsdig2 (Fig. 2). Then they were overlaid to extract the form data and remove non-form data such as size, position and direction by procrustes analysis (GPA) [13]. Body shape data were analyzed using multivariate analyzes, Principal Component Analysis (PCA), Canonical Variate Analysis (CVA) and Cluster Analysis (CA). The Mahalanobis distances were extracted among populations in CVA analysis. All the analyses were performed using Past and MorphoJ software.
3. Result
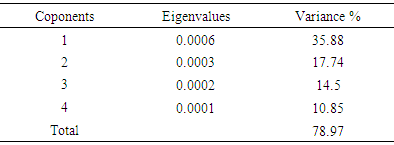
- In PCA analysis of the data, the first four principal components accounted for a total of about 79% of the variance. The amounts of each component are presented in Table 1. Change in the snout position is accounted for the highest percentage of the variance. Based on the results of PCA, there is no significant difference among the populations and they are overlapping (Figs. 3 and 4).
|
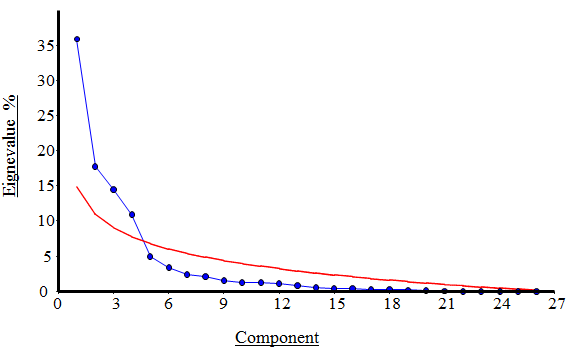 | Figure 3. Principal component analysis and the scatter plot of Joliffe cut-off point (red line), which represents the principal components significant border in populations |
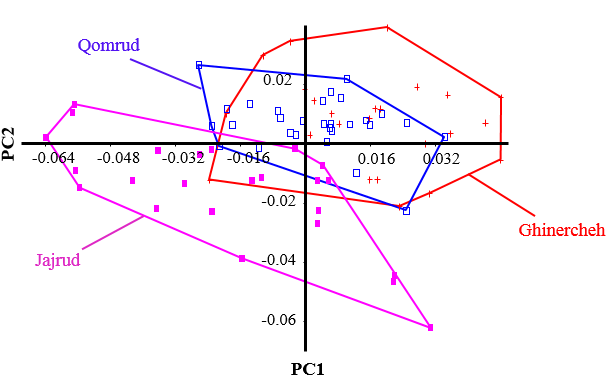 | Figure 4. Principal components analysis chart of body shape of chub (Squalius namak) populations in the rivers of Namak basin |
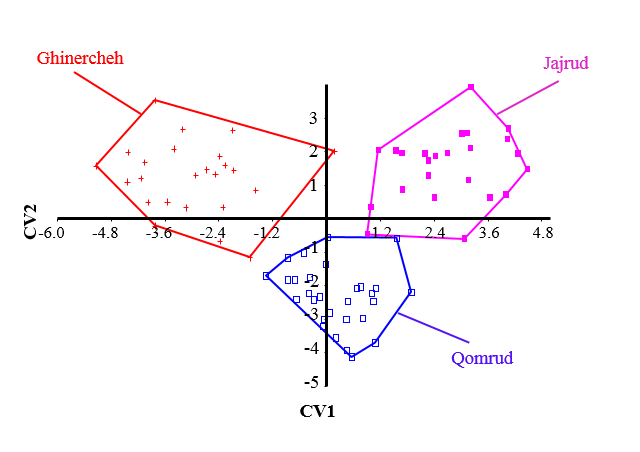 | Figure 5. Canonical Variate Analysis of body shape in Squalius namak populations in Namak basin |
|
|
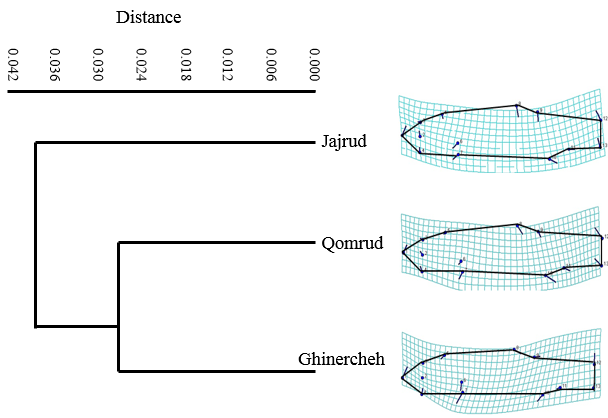 | Figure 6. Body shape cluster analysis in Squalius namak populations of Lake Namak basin |
4. Discussion
- The basic way to understand the aspects of fish biology and optimal management of resources is identifying the fish species and populations [14, 15]. Fishes are showing the most sensitivity to environmental changes among vertebrates [16]. Different environmental conditions cause changes and morphological differences among populations. These factors include the availability of food, water flow, turbidity and water depth [17]. The results showed that there were significant differences among populations in different habitats in Namak basin. These differences are related to position of the snout, head size and position of anal and pectoral fins. Hence, one purposes of this study was to evaluate and compare various body shapes in Namak chub populations. The results reveal differences in morphology of the populations of this species. Accordingly, Qomrud River population was tended to have a higher snout and shallower head. As well as Ghinercheh population is identifiable by a more inferior mouth, anterior anal fin and shallower caudal peduncle than other populations. Jajrud population is distinguished from other populations by a deeper body and a more posterior anal fin. This morphological attributes are affected by environmental factors or genetic differences during the developmental processes [8]. However, the formation of a feature is far slower than changes in the environment. So it could be said that the main morphological variability is a long-term solution to environmental changes [18]. Despite observed morphological differences among populations, there were also seen like those in the crowd in the chub. This can be indicative of features that enable the fish to live in different aquatic ecosystems. Change in the head and mouth may reflect differences in nutrition [19]. The shape of the mouth is a prototypical morphological feature for members of the species and despite the difference in length and width of head, they have maintained the position of the mouth, although its location is more ventral in Ghinercheh population, thus it is likely that this population feeds more from the bottom [21]. The observed differences in Squalius namak represents the morphological changes in relation to the habitat [11]. The morphological patterns showed that Ghinercheh River population has a shallower body that might be an adaptation to the faster water flow by prevention of washing [20]. Also the shallower head may be due to differences in food levels used [18]. As well as, changes in caudal fin length and width can be related to the enhanced performance in fast currents which is the main role of caudal fin [22].
5. Conclusions
- It could be concluded that Squalius namak is a morphologically variable species that lives in variable environments [23]. This adaptation in aquatic ecosystems is a result of having to compromise with hydrodynamic forces to save energy during bio-related behaviors. Morphological variability is not always indicative of environment and genetic differences of the populations might be involved. So also it is suggested to examine the populations with genetic and molecular methods [24, 25].
ACKNOWLEDGEMENTS
- We would like to thank Dr. S. Dorafshan, Dr. M. Nasri, Mr. S. Asadollah, Mr. A. Nezamoleslami and Mr. A. Mirzaei for their help in field work. Mr. Mazaher Zamani-Faradonbe, Isfahan University of Technology Ichthyology Museum, is gratefully appreciated for his help in laboratory works. This research was financially supported by Isfahan University of Technology.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML