-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
p-ISSN: 2325-002X e-ISSN: 2325-0038
2015; 5(2): 32-37
doi:10.5923/j.zoology.20150502.02

First Records of Butterfly Diversity on Two Remote Islands on the Volta Lake of Ghana, the Largest Reservoir by Total Surface Area in the World
Daniel Opoku Agyemang 1, Daniel Acquah-Lamptey 1, Roger Sigismond Anderson 2, Rosina Kyerematen 1, 2
1Department of Animal Biology and Conservation Science, University of Ghana, Legon, Ghana
2African Regional Postgraduate Programme in Insect Science, University of Ghana, Legon, Ghana
Correspondence to: Daniel Acquah-Lamptey , Department of Animal Biology and Conservation Science, University of Ghana, Legon, Ghana.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The construction of the Akosombo Dam in Ghana for hydroelectric energy led to the creation of many islands on the Volta Lake. The biological diversity on these islands is unknown and so a rapid assessment was conducted in January 2014 as part as a region wide assessment to determine the butterfly diversity on two of these islands, Biobio and Agbasiagba. Diversity indices were computed for both islands using the Shannon-Weiner index, Margalef’s index for richness and Whittaker’s index for comparison of diversity between the two islands. A total of eight hundred and eighty-one (881) individual butterflies representing forty-five (45) species belonging to eight (8) families were recorded during the study. Thirty-nine (39) species of butterflies were recorded on Biobio island whiles twenty-eight (28) species were recorded on Agbasiagba. This was expected as the larger islands are expected to support more species than smaller ones, with Biobio island being relatively bigger than Agbasiagba. The shared species of butterflies on both islands were twenty-two (22) representing 48.9% of the total species accumulated. Indicator species like Junonia oenone, Danaus chrysippus and Papilio demodocus were also recorded indicating the degraded floral quality of the Islands. Even though this survey has not found much, it serves as the first record of Butterflies on these Islands. A thorough assessment of these islands in a more suitable season is however being recommended.
Keywords: Butterfly, Diversity, Biobio, Agbasiagba, Volta Lake
Cite this paper: Daniel Opoku Agyemang , Daniel Acquah-Lamptey , Roger Sigismond Anderson , Rosina Kyerematen , First Records of Butterfly Diversity on Two Remote Islands on the Volta Lake of Ghana, the Largest Reservoir by Total Surface Area in the World, Research in Zoology , Vol. 5 No. 2, 2015, pp. 32-37. doi: 10.5923/j.zoology.20150502.02.
Article Outline
1. Introduction
- The order Lepidoptera is one of the most species-rich groups of insects, with an estimated number of species close to 146,000 [1]. Butterflies occur in all parts of the world, but they are primarily tropical. About 20,000 species of butterflies worldwide [2] and about 4,000 species have been identified so far from Africa [2], [3]. The butterflies of West Africa, especially Ghana are well studied [2], [4], and [5]. Currently, Ghana has about 925 species of butterflies [2], most of which have been described from the various protected areas and reserves [2] but generally there is limited knowledge of butterflies of the Volta Region of Ghana [1] where this research was carried out. Butterflies are useful insects and are of considerable economic importance. The larvae of most species cause damage to cultivated crops, stored grains and fabrics [6], [7]. However, the aesthetic beauty and “charismatic” nature of many butterflies have the ability to invoke people’s passion and interest, both of which are useful in butterfly conservation [8]. Butterflies serve as biological indicators which provide information about the health of an ecosystem; hence, they are used as model organisms to study the impact of habitat loss, fragmentation and climate change [1]. Member species show a diversity of relative sensitivities to environmental change, and they are interconnected with ecological systems as both primary consumers (herbivores) and as food items [9]. This attribute also makes butterflies good candidates in Rapid Assessment Program (RAP) surveys [1]. The construction of the Akosombo Dam (1961-1965) resulted in the creation of some islands on the Volta Lake which could serve as homes to several plant and animal species, including butterflies. Islands contain about 40% of all critically endangered species with extinction rates disproportionately greater than that of main lands [10]. It is therefore pertinent to study the biological structure and composition of life forms on islands. This is because it cannot be ascertained what useful resources, beneficial for human survival such as food, medicine or genetic resources remain untapped on unsurveyed islands. This study thus sought to provide baseline data on butterfly species identification and diversity on two islands – Agbasiagba and Biobio, created by the construction of the Akosombo dam.
2. Materials and Methods
2.1. Sampling Sites
- Agbasiagba Island which is about 13.97 km from the mainland lies southwest of Kpando in the Volta Region of Ghana on 6° 56' 33.2628'' N and 0° 9' 0.5005'' E with an altitude of 324-600ft. The island has an area of about 0.53km2 with an estimated population size of about sixty (60) people predominantly fishermen and farmers. The landscape is basically comprised of rocky outcrops with few thickets interspersed with Neem trees. Biobio on the other hand lies northwest of Kpando on latitude 7° 6' 6.8940'' N and 0° 11' 35.5380'' E with an altitude of 300-600ft and is 12.38km from the mainland. The island covers an area of about 0.79km2 with a population estimate of about 120-300 people. Biobio has more pronounced grassland vegetation interspersed with trees and the most encountered tree was Neem (Azadirachta indica), depicting the Savannah Woodland vegetation that is known to exist in the Volta Region. The two Islands are about 18.36km apart [11], [12]. Prior to visiting islands, permit was secured from the family heads who overseers the islands.
2.2. Sampling Methods
- Sampling was carried out using aerial nets; transect walk-and-counts and Charaxes traps. Although a minimum of three hours was spent during each sampling period twice each day using random walk sampling by three persons until all accessible areas on the island was covered, the total transect walk for each site remained the same. This was repeated for all representative habitat types. The Charaxes traps were baited with rotten banana mixed with alcohol (beer) and the target species were mainly alcohol-loving butterflies of the family Charaxidae which are fast flyers and difficult to identify in flight as well as other butterflies attracted to fermenting fruits. Six of these traps were randomly set about 100 metres apart on both islands and were left hanging for up to three days at a time with the bait recharged each morning. Trapped butterflies were killed in a killing jar charged with ethyl acetate and kept in labelled envelopes for later identification. Aerial nets were also used to capture some butterflies, especially those which were not easily identifiable in flight and also to confirm identification. Butterflies were identified in flight and recorded along demarcated transects along the islands. The thoraxes of butterfly specimen captured were gently squeezed to immobilize them and then placed in labelled envelops for later examination and identification. Easily identified species were released whereas the rest were identified with reference to the collections in the Biodiversity and Entomology Museum of the Department of Animal Biology and Conservation Science, University of Ghana, [4], [13], [14], [15], [16], [17], [18] and [19].
2.3. Data Analysis
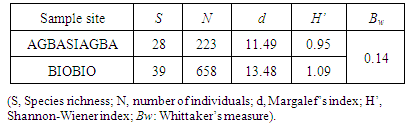
- Shannon-Weiner diversity and Margalef’s richness indices were computed for each island whereas Whittaker’s measure was computed to determine the diversity between the two islands. Thus, the diversity indices computed are:● Shannon-Weiner (H’ ) - -Σpi x In(Pi)● Margalef’s Richness (d) - (S – 1) / log(N)● Whittaker’s Measure (Bw) - (S/α)-1Where;S is the species number, N is the number of individuals captured, H’ is the species diversity within a community or habitat, Pi is the proportion of individuals of each species belonging to the ith species of the total number of individuals, α is the number of species per plot d is a simple measure of species richness and Bw is the amount of compositional variation between the two islands.
3. Results
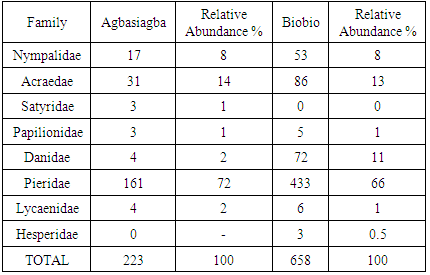
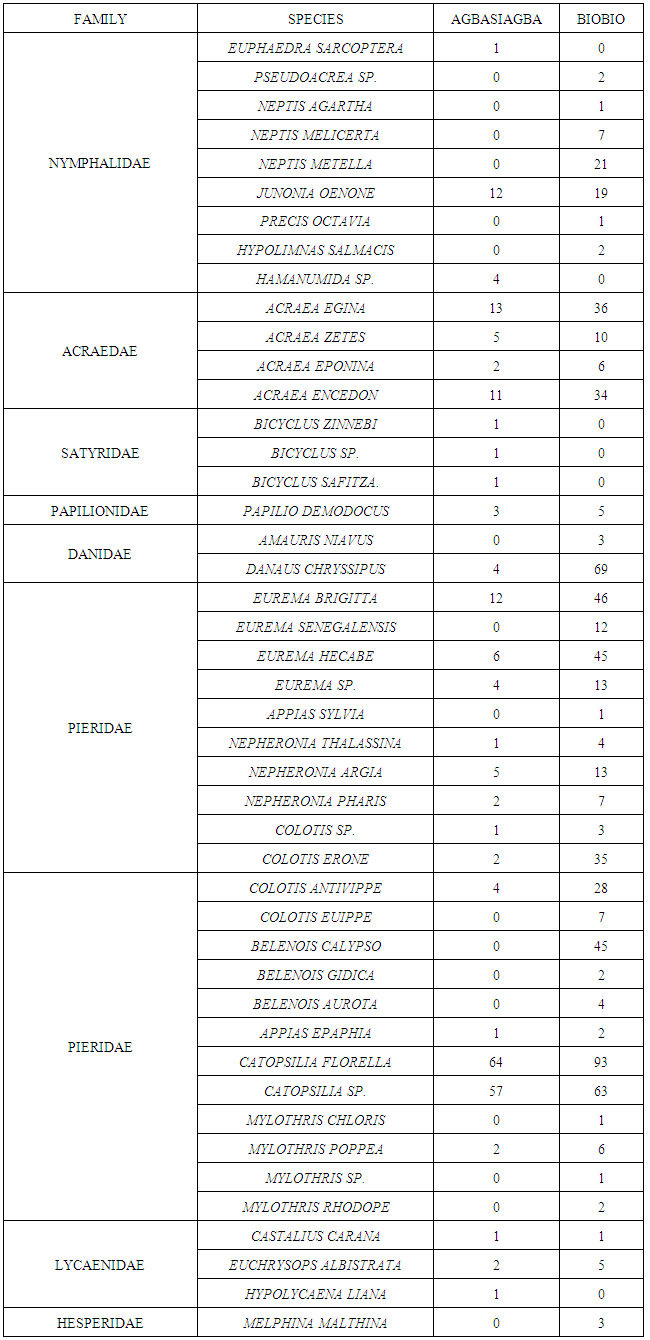
- A total of 881 individual butterflies representing 45 species belonging to eight (8) families were recorded; Acraeidae, Nymphalidae, Satyridae, Papilionidae, Danaidae, Pieridae, Lycaenidae and Hesperidae, with Pieridae dominating with 22 species. Seven butterfly families were recorded at Biobio with Pieridae dominating with 66% of the butterflies encountered (Table 1) whiles seven families were also recorded at Agbasiagba with the Pierids dominating with 72% of the butterflies encountered (Table 1).
|
|
4. Discussion
- The low species richness, diversity and abundance recorded for Agbasiagba could be attributed to the fact that vegetation cover was minimal at the time of sampling (January). There was also some evidence of burning. Although the main occupation of the islanders is fishing many also engaged in small scale farming basically for domestic consumption and so most of the land had been converted to farm lands. This study is in accordance with a study conducted [20] at the Muni-Pomadze Ramsar Site in the central region of Ghana where parts of the Ramsar site that had been converted to farmlands recorded the lowest abundance and diversity during the same sampling period. This was attributed to the fact that at the time of the survey in January; most of the vegetation cover at the Ramsar site had been removed by burning which was a common practice for that time of the year. A study conducted by [9] on butterflies of two forested sacred groves in the Eastern Region of Ghana however revealed that butterflies were more specious and diverse in the dry season most probably due to high temperatures.Biobio on the other hand recorded a higher species richness, diversity and abundance than that of Agbasiagba. This could most probably be attributed to the bigger size of Biobio as well as the relatively richer vegetation. Factors such as resource availability for adults and larval host plants, behavioural traits, and interaction with other species could explain the increase in butterfly diversity and richness on Biobio Island, though these factors were not adequately measured during this survey. The higher species diversity and richness on Biobio Island is consistent with the theory that large islands tends to support more species than smaller ones [21, 22] though the assertions cannot be confidently made due to the season the survey was conducted.The beta diversity between the two islands which was very low (Bw = 0.14) confirms a low variability in species composition between the two islands. This might generally be due to the time of the survey which was the dry season. There were twenty-two species common to both islands which represent about forty-nine (49) per cent of the total species population encountered on both islands despite the variation in habitat size. This is about half of the total species recorded and this might be due to the fact that both islands are found in the same ecological zone with similar climatic conditions which supports most of these species. The Pierids dominated on both islands probably due to the open nature of the vegetation which allows for free flight and easy movement [16]. The Lycaenids recorded a relatively low abundance on both islands. These butterfly species live mutually with their ant hosts. According to [23], many species of Lycaenids are regular hill-toppers. Poor accessibility to the hills might have accounted for the low numbers recorded during the survey. Moreover, these species are mymecophilus, thus are found near their ant host, the various species of Crematogaster observed were not abundant on the islands. Catopsilia florella which was the most abundant butterfly in the survey is a migratory species, and is commonly known as the African Emigrant. It usually occupies savannah country habitat, but can effectively colonize disturbed areas [24], Butterfly species such as Papilio demodocus and Junonia eonone are known to be much more common in West Africa now than they ever were due to the widespread destruction and fragmentation of forest cover that has taken place in this part of Africa [3]. These species recorded a higher abundance on Biobio Island than Agbasiagba which suggests degradation and poor vegetation quality although fragmentation and degradation were much higher on Agbasiagba than Biobio. This may be due to the availability of their food source on Biobio Island. Citrus sp. the main host-plant for P. demodocus, is common and widely distributed [4] and was abundant on Biobio Island. Junonia oenone, the Dark Blue Pansy, a nymphalid, is originally a savannah butterfly, but is now common in cleared areas of forest zones [4] The Lycinid Euchrysops albistriata, also known as Capronnier’s Cupid is widely distributed in the Guinea Savannah, slightly penetrating the Sudan Savannah, and in disturbed areas in the forest zone [4]. Danaus chrysippus, the Plain Tiger is originally a butterfly of the savannahs, but has successfully colonized disturbed habitats in the forest zone [4]. They were recorded in high numbers at Biobio indicating the presence of large portions of disturbed vegetation. Belenois calypso is widespread and common in most of the African forest zones, especially in somewhat drier areas, though it can be found around the edges of even the wettest forests. Males are often observed in mud-puddles, and both sexes are attracted to flowers [25] and this was fairly common on Biobio but absent on Agbasiagba Island.The presence of Papilio demodocus, Catopsilia florella, Eurema hecabe, Eurema brigitta, Mylothris chloris and Danaus chysippus is a clear indication of poor vegetation quality on both islands [3]. Butterflies, just like other insects too are able to conform to mild disturbances in their habitat [26, 27]. This was consistent with a Rapid Assessment Program survey conducted by [8] on butterflies on the Atewa Range Forest Reserve in Ghana; where Asiakwa South still recorded the highest number of species irrespective of the habitat fragmentation which had occurred there due to the exploration of bauxite mining and other illegal activities.
5. Conclusions
- This study is the first to be conducted on these islands and it provides a baseline data of butterfly species on the islands. From the results Biobio Island recorded a higher butterfly species richness and abundance than Agbasiagba Island. This was probably due to its bigger size and much more abundant resources in terms of vegetation cover. Although diversity was higher on Biobio, species distribution was similar on both islands and this could be attributed to the fact that these islands are in the same geographical zone with the same climatic conditions and they are not very distant from each other. These findings are not conclusive enough considering the fact that the survey was conducted in the dry season. It is however recommended that a thorough survey be conducted on all the Islands on the Volta Lake to properly document butterfly species as well as other biological fauna found on them and also to ascertain if any are of conservation value and thus warrant urgent conservation interventions and most importantly during the wet season as well to provide a better inventory of the species composition on these islands. This baseline data on Butterfly diversity when completed would enable the comparison with main land species to access if there has been any variation in species composition, structure or distribution between the islands and the adjoining main land.
ACKNOWLEDGEMENTS
- The authors are extremely indebted to the Chief and Elders of Agbasiagba and Biobio, who granted access to the Islands. Mr. Eric Cudjoe and Mr. Ackah were also instrumental in the success of this work and for that we are very grateful.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML