-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
p-ISSN: 2325-002X e-ISSN: 2325-0038
2015; 5(1): 16-19
doi:10.5923/j.zoology.20150501.02
Hematological Characteristics of Two Teleost Fish in a Tropical Reservoir
Felix O. Akinwumi
Department of Environmental Biology and Fisheries, Adekunle Ajasin University, Akungba-Akoko, Nigeria
Correspondence to: Felix O. Akinwumi, Department of Environmental Biology and Fisheries, Adekunle Ajasin University, Akungba-Akoko, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Theblood condition of the fish is crucial in meeting the health, food, economic and post-harvest needs of fish farmers and consumers. In this study, the hematological conditions of live and sacrificed samples of two commercially important teleosts, Clariasgariepinus (African mud catfish) and Channaafricana (Snake head fish), obtained from Egbe dam located in Ekiti State, southwest Nigeria were studied. Blood samples were collected from tranquilized live fish by puncturing the anal fin vein using 23G x 11/4” sized heparinized plastic syringe and later sacrificed by percussive stunning for post-mortem blood sample collections. Standard hematological analytical procedures were used in the assessment of the various blood parameters. The results obtained evoked significant differences (P<0.05) in the Packed Cell Volume (PCV), White blood Cell Counts (WBC), Red blood Cell Counts (RBC), Hemoglobin (Hb) and Blood platelet (PLT) of the live samples of the two teleosts. C. gariepinus had higher values of blood parameters profile than C. africana in all the analytical assessments with a higher 2:1 WBC counts, suggesting a lesser environmental and pathological stressors for C. africana at the time of sampling. There were no statistical differences (P>0.05) in the blood Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), and Mean Corpuscular Hemoglobin Concentration (MCHC) of the two live fish species. The results of the post-mortem analysis of the blood parameters revealed significant differences (P<0.05) at the different intervals of blood collection with the highest rate of decline in the blood profile within the first 2hours of death. Rigor-mortis had no visible impact on the decreasing status of the blood profiles of the two teleostean fish because the muscle stiffening was delayed for about 6hrs after the initialization of the post-mortem study. The results obtained in this study will serve as baseline hematological parameters for use in monitoring of fish health and preservation.
Keywords: Post-harvest, Freshwater, Fish, Post-mortem, Hematology
Cite this paper: Felix O. Akinwumi, Hematological Characteristics of Two Teleost Fish in a Tropical Reservoir, Research in Zoology , Vol. 5 No. 1, 2015, pp. 16-19. doi: 10.5923/j.zoology.20150501.02.
Article Outline
1. Introduction
- Fish is one of the potential sources of animal protein and essential nutrients for maintenance of a healthy body [1]. In recent years, fish have become a favourite foodstuff for the majority of societies because of several health reasons [2]. Water, protein, lipid and carbohydrate and mineral elements, such as, sodium, potassium, calcium, magnesium, phosphorus, sulphur, iron, chlorine, silicon, manganese, zinc and iodine are also commonly found in fish. The nutritional composition of fish varies greatly from one species and strains to another; depending on age, feed in-take, sex, the environment and seasons [3]. Blood, one of the principal components in fish, aids the normal physiological balance, regulates the various metabolic activities, maintains the acid-base balance and removes the metabolic waste products from tissues. The knowledge of haematological characteristics of fish gives the idea of their physiological status in relations to various environmental factors. The determination of blood parameters and in particular those of the red blood cell counts are used for assessing fish health [4]. Fish are known to live in close relationship with their environment. Studies have shown that variations in the blood composition of fish depend on the stress effects of the environmental factors [5]. In situations where environmental conditions are not favorable or there is an environmentally induced pathological condition, the blood will reveal conditions within the body of the fish, prior to the visible manifestation of a disease [6]. It has been reported that stressors in the environment can exert some influences on fish hematological characteristics [7]. Since the health of the fish is an important consideration for fish consumers, it is necessary to assess its blood characteristics during capture, processing and preservation. This study was designed to provide baseline scientific information about the blood values of two commercially important fish species in a tropical reservoir, Egbe Dam in Ekiti State, Nigeria. Information about the hematological characteristics of the fish species in the dam is just growing and the data obtained will improve the existing scientific database on fish hematology in Nigeria.
2. Materials and Methods
2.1. Sample Collection
- Samples of Clarias gariepinus (African mud catfish) and Channa africana (Snake head fish) were collected from Egbe Dam (latitude 7°36’N and longitude 5°36’E) in Gbonyin Local Government Area of Ekiti State, Nigeria. The fish were captured with basket traps set overnight under the vegetative part of the dam. Captured specimens were transported early in the morning (between 7:30am and 9:00am) to the laboratory for blood sample collection. Specimens used for blood sample collection were adult fish with the average total length of 19.0-to-25cm.
2.2. Blood Collection and Processing
- Blood samples were first collected from live fishes tranquilized with 150mg L-1 solution of tricane methanesulphate (ms-222). This was done through intravenous withdrawal by puncturing anal fin vein using heparinized plastic syringe fitted with 23 x 11/4”-guage hypodermic needle. The fish were then sacrificed by percussive stunning for post mortem blood sample collections. Three regimes of post-mortem blood sample collections were observed at 0-30 minutes; 2hours-2:30hours and 4hours-4:30hours). All blood samples collected were preserved in Ethlyne Diamine Tetra-Acetic Acid (EDTA) sample bottles. Standard hematological analytical procedures were employed in the assessment of the blood parameters. The micro-hematocrit method was used for the determination of the packed cell volume (PCV) [8]. Hemoglobin (Cyamethemoglobin) and red blood cell concentrations were determined by the methods of [9] while the white blood cell (WBC) count was determined by Neubauer hematocytometer [10]. The derived hematological indices of Mean corpuscular volume (MCV), Mean corpuscular hemoglobin (MCH) and Mean corpuscular hemoglobin concentration (MCHC) were calculated using the standard formula, MCV = PCV/RBC X 10; MCH = Hb/RBC X10 and MCHC = Hb in 100mg/ PCV X100. Data analysis was performed using SPSS for windows 17.0 package program. Analyses of variance were carried out at 0.05 significance level.
3. Results and Discussion
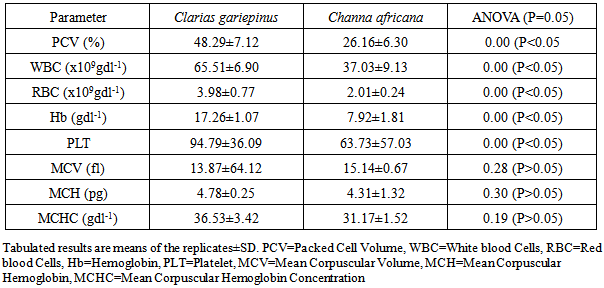
- The results of the laboratory assessment of blood parameters of the live samples of C. gariepinus and C. africana collected from Egbe dam are presented in Table 1. It was observed that significant differences existed (P<0.05) in the Packed Cell Volume (PCV), White blood Cell Counts (WBC), Red blood Cell Counts (RBC), Hemoglobin (Hb) and Blood platelet (PLT) of the two fish species. C. gariepinus had higher values of blood parameters than C. africana irrespective of sex, body size and weight. However, there were no significant differences (P>0.05) in the blood Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), and Mean Corpuscular Hemoglobin Concentration (MCHC) of the two fish species. Age, sex, body size and weight influenced the hematological status of fish [11]. The results obtained in the study showed that the majority of C. gariepinus samples collected had a higher condition factor than C. africana. This may be indicative of a better ecological well-being for the Clarias species in the dam ecosystem than for the Channa species. In disease diagnostics, a higher WBC count is usually positively correlated to the immune system stimulation for defense against diseases and environmental stressors [12, 13, 14]. The lowercount of WBC (37.03±9.13x109gdl-1) obtained in the live specimen of C. africana in comparison to the higher profile (65.51±6.9 x109gdl-1) exhibited by C. gariepinus suggests that C. africana could be less susceptible to environmental and pathological stressors at the time of sampling because of the lower WBC counts. Leukocytosis was observed to be directly proportional to severity of stress condition in maturing fish and that the condition was a direct stimulation of immunological defence due to the possible presence of environmental stressors [15]. The higher RBC (3.98±0.77 x109gdl-1) and Hb (17.26±1.07x109gdl-1) profiles of C. gariepinus compared to C. africana (2.01 ± 0.24 x 109 gdl-1; 7.92±1.81x109gdl-1) might result to a higher energy production and the ability to adapt better to physiological stress, such as, high temperature and lowered dissolved oxygen level for C. gariepinus, especially, during dry season in the tropics.
|
|
|
4. Conclusions
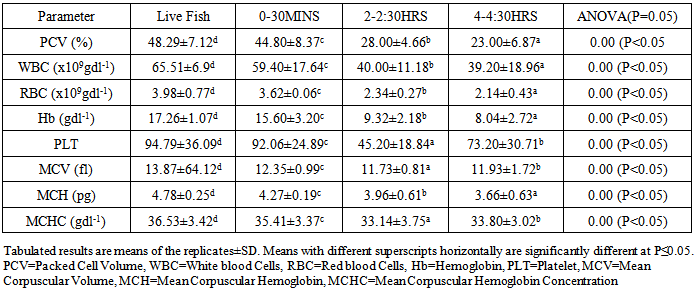
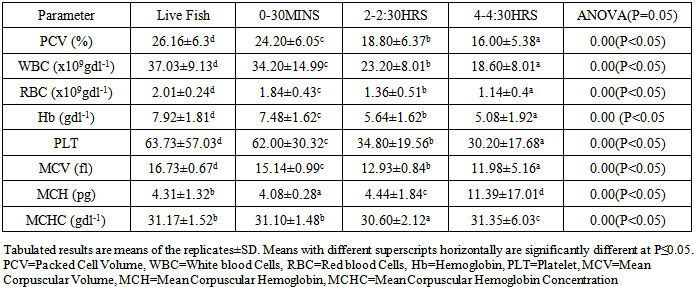
- This study showed that the hematological parameters of the studied fish species diminished significantly within the first 2hours while rigor-mortis was delayed. The results obtained in the study revealed that the autolytic reactions on the blood of the fish species presumed rigor mortis. The low profile of the blood level in C. africana is suggested to be responsible for its faster diminishing rate compared to C. gariepinus which had a higher blood profile. It is recommended that the immediate consumption of freshly harvested fish and or their appropriate processing and storage techniques are critical in fish utilization.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML