-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
p-ISSN: 2325-002X e-ISSN: 2325-0038
2015; 5(1): 1-15
doi:10.5923/j.zoology.20150501.01
The Regulation of Ecological Communities Through Feedback Loops: A Review
Luis Soto-Ortiz
Department of Biomedical Engineering, University of California, Irvine, California, USA
Correspondence to: Luis Soto-Ortiz , Department of Biomedical Engineering, University of California, Irvine, California, USA.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The aim of this review article is to emphasize the valuable contribution of theories describing the mechanisms that regulate communities, by describing how they have served to highlight the role of feedback in nature. The objective of early experimental work was to identify the mechanisms that could better explain the emergent population dynamics, especially the coexistence of competitors and of predator and prey. Some proposed explanations have gone in and out of favor as the wealth of experimental evidence has increased. Some researchers concluded that competition is the single most important interaction between organisms. Others believed that top-down effects, such as predation, are the most relevant, while still others championed bottom-up effects, symbioses or parasitism. In retrospect, it appears that these and other hypotheses share a common thread: the regulatory effect of feedback loops in communities. Feedback mechanisms are ubiquitous in nature, and the application of control and dynamical systems theory has helped to unify previously conflicting hypotheses into a theory based on systems ecology. This unification has enhanced our understanding of the extent to which regulatory feedback mechanisms influence community and ecosystem dynamics.
Keywords: Community, Systems Ecology, Feedback, Input-Output Control
Cite this paper: Luis Soto-Ortiz , The Regulation of Ecological Communities Through Feedback Loops: A Review, Research in Zoology , Vol. 5 No. 1, 2015, pp. 1-15. doi: 10.5923/j.zoology.20150501.01.
Article Outline
1. Introduction
- The exponential law of population growth, known as the Malthusian Principle [1], is one of the earliest proposals on population dynamics. It states that all populations of organisms have the potential to grow or decline exponentially in the absence of external forces. To Malthus, this type of growth seemed to be the default state of all species in nature, and would inevitably lead to a “difficulty of subsistence” if the reproduction of these organisms were not checked. The fact that organisms tend to produce more offspring than the environmental resources can sustain is known as the Malthusian dilemma. It explained why population explosions or outbreaks were not observed as hypothesized. Pierre-Francois Verhulst proposed that intraspecific competition was another force controlling population growth [2]. Influenced by these ideas, Charles Darwin introduced the concept of natural selection to explain how organisms evolve over time due to the inevitable struggle for existence [3]. Eventually, natural selection was considered to be the most important regulatory mechanism. Natural selection was considered to be instrumental in keeping populations in check. It explained why a dominant species could locally exclude a competitively inferior species and described how new species arose [3]. To Darwin, the extinction of some species seemed to be the normal way in which a finely tuned system should operate. The survival of the fittest would be the typical outcome of a natural process. Georgyi Gause provided experimental evidence that supported Darwin’s contention that the struggle for existence tends to be more intense between conspecifics, or between organisms sharing very similar resources. His experimental data on paramecia showed that, once one of the species monopolizes the common resources, the two populations reach a maximum density and one species will inevitably displace the other. Gause’s experiments led to the development of the competitive exclusion principle [4].In subsequent years, the phenomena that puzzled biologists and ecologists was the coexistence of competitors, the existence of cooperative interactions, the persistence over time of predator-prey interactions (often cyclic), and the fact that in certain interactions the competitive advantage varied over temporal or spatial scales, depending on the environmental parameters [5]. The empirical evidence seemed to contradict the competitive exclusion principle. These confounding observations led to more intense research and better-designed experiments to determine the cause for coexistence. In the process of trying to solve this mystery, ecological explanations emphasizing competition, keystone predation, niches, and fluctuations in prey recruitment and productivity, were proposed but they did not explain coexistence completely. Subsequent experiments demonstrated that the coexistence between predator and prey is a complex situation that could be more fully explained by a combination of the aforementioned theories [6]. Previous experimental results [7-9] and mathematical modeling [10-13] provided credible evidence that feedback mechanisms act as a regulating force in ecological systems, an idea proposed early on by Patten and Odum [14]. Feedback loops are the driving force that make possible the persistence of exploitative and mutualistic interactions, including those formerly explained by theories championing the effects of competition, predation, cooperation, niches, bottom-up effects, or other biotic / abiotic factors. These regulatory mechanisms influence ecological communities, and their effect can be better determined by applying control and dynamical systems theory.In this review article, the concept of feedback control is first illustrated through an intuitive example: the cruise control of an automobile. Then, control theory is applied to describe regulatory mechanisms found in ecological communities. Several mechanisms of population control proposed by ecologists are then described, while highlighting the important role that positive and negative feedback plays in the interactions between species. The discussion section emphasizes the contribution of mathematical modelling and systems ecology to our understanding of natural phenomena, while pointing out some of the challenges that still remain.
2. System Control via Feedback
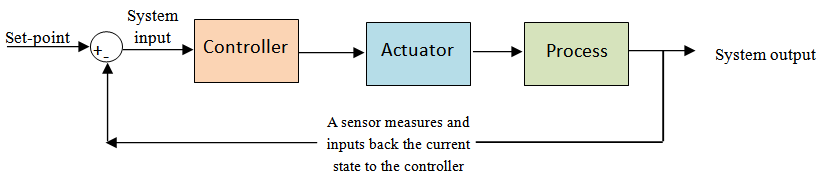
- Using fundamental notions of control theory as a starting point, feedback has been used to control the dynamics of electrical, mechanical and chemical systems. In this section, I will first describe the elements of a prototypical system controlled via feedback, coined as a cybernetic system by Wiener [15]. Then, I will discuss a real-life application involving the cruise control system of a vehicle to illustrate the effect of positive and negative feedback, both of which play an important role in this mechanical system and also in ecological interactions.Given a general input-output system, the elements of feedback control include the following:1. a process or plant, to be controlled, 2. a sensor that can directly measure or estimate a subset of the output of the system,3. the portion of the output that we are interested in measuring is the state of the system,4. a loop that “feeds back” the information on the state of the system, gathered by the sensor, as an input to the system, 5. a controller that receives the input and determines what action to take in order to remain as close as possible to the desired state (the set-point), and6. an actuator that carries out the action commanded by the controller.The feedback loop process repeats itself to maintain the state of the system at the desired set-point. Figure 1 depicts a process controlled by feedback.
3. Feedback Control in Communities
- The Malthusian Principle, by its assumption that all organisms tend to increase exponentially, is an example of positive feedback on population size. Suppose there exists a species that has unlimited resources (food, space, etc.) and that experiences little or no mortality. The density of this species will increase exponentially without bounds (a runaway process) due to the effect of positive feedback on the growth rate. If we consider this population to be an ecological input-output system, then, based on an analogy to a mechanical system:• The process to be controlled is the population growth rate. • The population density is the state of the system at a particular time.• The number of new zygotes after a reproduction cycle is the output of the system.• The growth of zygotes into new recruits to the population is the feeding back or input to the system. This assumes that the zygotes are able to survive the early stages of the life cycle and stay within the population.• The controller can be a density-dependent mechanism (competition, predation, cannibalism, etc.) or a density-independent mechanism (external disturbances such as changes in environmental conditions) that suppresses or promotes population growth.• The actuator can be either the reproduction process by the adults already present in the population in the previous reproduction cycle, along with the recent recruits that have reached the reproductive stage and are producing zygotes (this is a positive feedback on growth rate), or the mortality effected by competition, overcrowding, or disease (this is a negative feedback on growth rate).In general, ecological systems possess the elements just described and, consequently, are subject to regulation via feedback loops. Therefore, it can be concluded that any positive population growth rate observed in nature can be attributed to feedback loops present in the system that promote an increase in growth rate. Whether the population continues to grow indefinitely depends on whether the assumptions continue to hold, i.e. resources are unlimited and mortality is negligible.In nature, we seldom observe populations grow exponentially without bounds. Limiting factors and environmental disturbances are usually present in ecological systems, and they account for the decrease in population growth. As positive feedback continues to amplify the growth rate, the resources tend to diminish to a level below which a limiting factor (food availability, space, etc.) begins to exert negative feedback on the growth rate. This is an example of a density-dependent regulation [21 – 23], where negative feedback reduces the rate of increase in population density. The density of a population may level off and asymptotically approach a stable equilibrium value (the carrying capacity of the environment), or it may fluctuate periodically or chaotically around a mean value.Interdisciplinary collaboration between researchers in different fields has led to the consensus that it is possible to unite, via control theory and systems ecology, the major hypotheses that tried to explain natural ecological phenomena. In retrospect, these hypotheses actually described different types of ecological controllers that may be present in communities and that are responsible for the regulation of population density, the local exclusion of a species by a competitor, the coexistence of competitors, the coexistence of predator and prey and mutualism. Therefore, it is possible that contrasting points of view, as well as contradictory experimental evidence, can be integrated into a single unifying proposal that considers populations as systems subject to feedback loops, whose dynamics are governed by multiple controllers, and that are influenced by external perturbations such as abrupt changes in environmental conditions.
4. Ecological Controllers
- I will now describe, from the point of view of control theory, some of the major theories that have been proposed to explain the emergent population dynamics of certain communities. The key aspect of these theories is the fact that they all describe some controlling mechanism responsible for the regulation of communities. In this context, the operational definition of a community is the biological structure in an ecosystem comprised of the intra- and inter-specific interactions between species at the same, or distinct, trophic levels.
4.1. Competition Theory
- Ecologists have not always agreed on the prevalence and importance of intraspecific versus interspecific competition for resources in interactions among organisms. Darwin proposed that competition would be most intense between conspecifics [3]. Some ecologists have provided evidence of the relative importance of intraspecific competition [24-28] and density-dependent intraspecific aggression independent of food availability [29]. On the other hand, other researchers believed that there is significant evidence that supports the conjecture that interspecific competition plays a major role in certain interactions [30-32]. Unfortunately, disagreements on the importance of competition as a strong regulating factor persisted. Empiricists tended to believe that they could prove a theory by appealing to experimental evidence that supported it, but others argued that the best experimental procedure to follow was the strong inference protocol, which emphasizes forming and testing alternative hypotheses [33]. Strong inference has been proved to be helpful in determining the importance of underlying factors that had not been considered before, and experiments that employed strong inference have been instrumental in understanding complex food webs.Whether strong inference is applied or not when conducting experimental work, it is certain that any type of competition, if present in an ecological system, acts as a controller within the feedback loop regulating the population dynamics of communities. Competition slows down the process of population growth, and may even reverse the direction of the process, i.e. the mortality rate becomes greater than the birth rate. If competition leads to an increase in adult mortality, the number of new recruits (input to the system) will decrease in the next generation, and there will be fewer individuals reproducing in the next spawning season than there would have been otherwise. The intensity of competition, and consequently, the effect of negative feedback, may be strong or weak, but it will be present nevertheless. Therefore, we can generalize that this ecological interaction becomes a system subjected to some level of negative feedback. It would certainly be enlightening to understand what type of competition is having the greater effect. It may turn out to be that interspecific, rather than intraspecific, competition is the more intense and prevalent of the two [31], or vise versa [34]. It could be that in certain interactions, predation affects population density to a greater extent than competition, but both mechanisms are present and influence the overall regulatory effect. More insight is gained by first looking at a community “from the outside” via a systems perspective, i.e. by analyzing the inputs and outputs of the community, and noticing how the respective subsystems affect each other, rather than applying a reductionist approach where we fix our attention only on the inner mechanisms of a particular subsystem. By understanding that the input and output depend on the type of feedback, as well as how strong the feedback is, we can analyze a community by following the circuitry (analogous to the interactome in molecular biology) and determining which are the dominant controlling mechanisms in the network of species interactions. Even though ecologists have not fully reached a consensus on the most important and prevalent types of ecological controllers, there is widespread agreement that a typical ecological system is often subjected to inner forces and outer disturbances that act as regulatory mechanisms. Nature can be modeled as a set of linked dynamical systems that evolve over time and that affect each other, and this perspective has provided a better understanding of biological and ecological phenomena.
4.2. Predation Theory
- Keystone predation [35, 36] and diffuse predation [37, 38] have been identified as another kind of ecological controller that is embedded in a feedback loop. This controller can have a strong influence on the age structure, spatial distribution, and density of the prey and its competitors. Predation is a top-down process that has a cascading effect on lower trophic levels. Solomon [39] identified two kinds of behavioral predatory response to changes in prey density, which he labeled as the numerical and the functional responses. Keystone predators that are capable of a numerical response [40] or a functional response [41] exert negative feedback on prey density and can determine the variety of species in a community. These predators may aggregate in larger numbers, or increase their prey consumption, in response to increases in prey density. If prey density is low, predators may disband and forage in areas of greater food availability, or they may simply reduce their attack rate. Therefore, an increase in prey density leads to negative feedback on the prey population via increased predation, which will decrease either prey density or prey growth rate. In addition, a low prey density may decrease predation and effect prey growth, since predators reduce their attack rate in response to scarcity of food and are unable to inhibit the growth of the prey population. Consequently, positive feedback on prey growth manifests itself through the law of exponential growth. A loop is created such that the predators are able to regulate the density of prey through their behavioral responses, analogous to the way in which the already described cruise control feature stabilizes the speed of a vehicle. Another aspect of predation to consider is that a predator that possesses a physiological developmental response [42], can increase the effect of its negative feedback on the prey population as the predator grows, since larger predators experience lower prey-handling costs and have a greater predation efficacy. It is worth noting that keystone predation can also involve positive, rather than negative, feedback as a regulatory mechanism of prey biomass [43].Keystone predation can lead to higher species diversity through negative feedback on the population of the dominant species, which may be the preferred prey of the keystone predator. Consequently, the dominant species experiences a higher mortality rate. Other species, free from the competitive advantage of the dominant species, can reproduce successfully and can settle in areas where the dominant competitor had previously excluded them. In the presence of the dominant competitor, the less competitive species experience strong negative feedback on their growth because the dominant species monopolizes the common resource. On the other hand, when the density of the dominant species is significantly reduced, the weaker competitors will increase their density, and may increase their spatial range. Therefore, it has become clear that predation can lead to a regulatory mechanism involving negative and positive feedback loops [44] between linked subsystems (competing populations in this case) that increases species diversity [35]. The reason why the population size of a dominant species does not grow indefinitely when predators are not present, may be due to the fact that as the density of the dominant species increases, intraspecific competition for the diminishing resources increases, along with disease and/or overcrowding, leading to an increase in the mortality rate (a case of negative feedback via intraspecific competition). As empirical evidence accumulated over the years, it became evident that a population is a subsystem of a larger community, that each subsystem experiences both positive and negative feedback via predation and competition, and that the subsystems in a community are connected through a series of inputs and outputs that act as direct or indirect sources of feedback that affect other subsystems. A predator that possesses a numerical, functional, or developmental response will increase the attack rate on its prey, and in so doing, will cause a decrease in negative feedback experienced by the competitors of its prey. This will increase the density of the competitors until intraspecific competition, disease, or overcrowding, stabilizes their densities via negative feedback. An insight that has been achieved through the application of control theory to study ecological systems is that if the population dynamics of a community tend toward asymptotic stability, then all of its subsystems must also tend toward stability. That is, if the total density of a metapopulation is approaching the carrying capacity asymptotically, then each local species density within the system must also be approaching its own particular equilibrium value. Diffuse predation, which may or may not involve a keystone predator, occurs whenever groups of predators of different species unite forces to stabilize the population of a common prey. It has been shown that if one group of predators is removed, the other group of predators compensates for the absence of the first group by increasing its density to keep the prey population in check. Thus, in diffuse predation, the strength of the negative feedback exerted by the predators on their common prey remains constant due to the compensatory effect of diffuse predation. Compensatory predators experience negative feedback themselves, due to interspecific competition for common resources. When a compensatory predator species is removed, or its density decreased, the competition tends to decrease, and allows the other group of compensatory predators to increase their density. Therefore, the controller exerts the same regulatory effect on the population growth of the common prey due to the compensatory control exerted by diffuse predation.The fundamental importance to food web dynamics of scavenging in vertebrate predator –prey interactions has been highlighted by the emergence of multiple indirect top-down effects of carrion and facultative consumption by predators [45]. Tropic cascades affect arthropod composition due to intra-guild predation between competing vertebrate predators where a predator kills and consumes a potential competitor [46]. Feedback also plays a major role in plant-herbivore interactions. Many plants have evolved defense mechanisms allowing them to release chemicals to ward off potential attacks by predators. Through a feedback loop, the defense mechanisms of a plant are increased when herbivory is intense, and are decreased when the rate of predation decreases significantly or stops completely. Through inducible defense mechanisms, a plant is able to avoid unnecessary maintenance costs and can adapt to changes in predation rates.
4.3. Niche Theory
- An example of an ecological theory that went in and out of fashion, but proved to be influential, is classical niche theory [47]. This hypothesis sought to explain the coexistence of competitors and was eventually modified and labeled as contemporary niche theory. The niche of an organism can be defined as its lifestyle: what it eats, where it lives, when it feeds, how it gets its food, when it reproduces, etc., and how it impacts the environment [48]. The adoption of a niche as an evolutionary adaptation to competition assumes a genetic change, and is not simply a temporary phenotypic adaptation. Proponents of niche theory used it to explain why competitors can coexist by describing how they reduce their niches to decrease the negative feedback caused by interspecific competition. In addition, a single population may experience strong intraspecific competition and will expand its niche until it genetically diverges into two new species that share few, if any, resources. This allows the species to initially increase their densities via the law of exponential growth. Eventually, each population or subsystem will approach equilibrium and the whole community will stabilize.Ecologists who advocated classical niche theory used it to explain why a species may diverge genetically and evolve into several species that adopt different lifestyles. Experiments have demonstrated that in some ecological systems, intraspecific competition can have a greater regulating effect on a population, than interspecific competition [34]. Coexistence of competitors is possible if each competing species suppresses itself more than it suppresses the density of its competitor [26]. That is, coexistence is possible whenever the effect of negative feedback caused by intraspecific competition is greater than the effect of negative feedback caused by interspecific competition. However, ecologists who supported niche theory did not completely agree on the cause of niche differentiation, as some believed that niches are influenced by adaptation to physical or environmental factors [49, 50], while others believed that niches and character displacement are an outcome of competition [51, 26]. Peter Grant [52] suggested that environmental factors and competition should both be taken into consideration to gain a better understanding of niche formation. The fact that competing species are able to coexist has been attributed to hydrological niche segregation, which has been observed to be a widespread phenomenon in terrestrial plant communities [53]. The existence of niche availability and niche discordance is thought to lead to ecological opportunity, which makes possible the survival of a species within a community, leads to divergence via diversifying selection within a lineage and promotes biodiversity [54].Feedback theory can be postulated to unify the different arguments as to the origin of niches. As a population evolves over time, it reduces the negative feedback commanded by the controller (in this case intraspecific competition), by partitioning itself into smaller subsystems, each composed of a single, newly evolved species that has its own particular niche. These populations were probably strongly linked in the past when they depended on similar resources and had similar lifestyles, but due to competition, they have evolved into weakly linked systems that now experience little or no interspecific competition. These new systems now regulate themselves as separate entities, and experience negative feedback mostly through intraspecific competition, which regulates their densities. Species that evolved in allopatry and adopted different niches separately, will not experience strong interspecific competition if brought together in sympatry. Niche theory advocated that regardless of whether populations live in allopatry or sympatry, they will evolve into weakly linked subsystems that are not sources of negative feedback for each other. In allopatry, each subsystem is self-regulating and, if brought together in sympatry, they will form a self-regulating community.
4.4. Supply-Side Theory
- Supply-side theory holds that the input of new recruits into a population plays an important role in determining the structure of the population [55, 56]. For example, a supply-side process may include the settlement and survival of larvae. Experiments have shown that fluctuations in larval recruitment tend to occur in mussel populations in the intertidal zone [35]. Larval recruitment, by definition, represents an input to an ecological system. Therefore, the level of larval recruitment will influence the intensity of positive feedback on the population. It has been shown that some larvae tend to settle passively [57-59], and that hydrodynamic stress may determine their distribution and lead to fluctuations in larval settlement [60, 61]. Other experiments have demonstrated that some types of larvae can actively move and are able to respond to physical or chemical cues, and consequently, they preferentially choose an attachment site [62-65]. Whether settlement is passive or active, larval mortality may be a function of distance from the shore. If larvae are passively drifting away from the shore, they may be able to disperse to other areas, and may even increase their survival rate, but they will not become an input (new recruits) to the local population. On the other hand, if larvae can actively swim and stay close to the shore, they may serve as input to the local population. Unfortunately, by staying close to the shore they may run a higher risk of predation [66]. Post-settlement mortality of larvae is also a factor that affects the actual input of new recruits to an ecological system. Therefore, due to hydrodynamic forces that randomly disperse larvae, and to random mortality of larvae due to grazing, the input to the system is subject to stochastic fluctuations. That is, the output of the system (all of the new zygotes) is not completely fed back into the system. Possible new recruits are lost due to pre-settlement mortality [67], or are lost due to random advection processes [61, 68-71]. Both cases are likely to occur if the larvae are meroplankton that live for longer periods in the water column [67, 72-74]. In control theory, white noise is a theoretical representation of a random process that affects how well sensors can read and feed back the output back into the system. The negative effect of this random disturbance manifests itself in the output and the performance of the system. A similar situation occurs in ecological systems: In the presence of random or “noisy” recruitment, the positive feedback may not be as intense as it could be if all the larvae were to survive, grow, and reproduce, and the population growth rate may not be as high as it could be. In some systems, variation in larval recruitment is common and does not depend on extreme disturbances. Therefore, in systems where supply-side effects play a major role in the distribution of a particular species, the effect of positive feedback on this system will vary over time due to the random processes affecting recruitment rates. In addition, it has been shown that predation rates may depend on spatial variability of prey recruitment over environmental gradients [75]. An increase (decrease) in prey recruitment may increase (decrease) the intensity of negative feedback caused by predation, if predators can respond to prey recruitment through behavioral or physiological mechanisms. Hence, fluctuations in prey recruitment can increase or decrease the intensity of positive feedback on the growth rate of prey, and this will affect the negative feedback on prey growth rate caused by predators.
4.5. Other Proposed Regulatory Mechanisms
- In addition to competition, predation, niche, and supply-side theories, other factors such as bottom-up [76, 77] and cooperation effects [78-81] that influence population dynamics have been proposed. In addition, controlled experimental manipulations have shown that top-down and bottom-up effects are both capable of influencing the density of a particular population [82, 83].Bottom-up effects are present when environmental disturbances, such as El Niño-Southern Oscillation (ENSO) events, alter primary production and species diversity, whether marine or terrestrial [84-86]. It is important to take into account bottom-up effects, which can be a source of negative feedback for species that belong to higher trophic levels when species in the lower trophic levels become depleted. On the other hand, a pulse of resources can lead to a strong positive feedback that increases the density of species in higher trophic levels [87]. Regulatory interactions acting between multiple trophic levels are common in nature. When primary production is diminished, herbivores are affected and their densities decrease due to competition for resources. Carnivores that depend on herbivores are also affected in a similar fashion. Therefore, bottom-up effects may lead to increased competition for resources in systems at higher trophic levels. If the magnitude of this negative feedback becomes greater than the positive feedback due to reproduction, population densities of species at these trophic levels will decrease over time. It has also been demonstrated in theory, as well as through a bottom-up effect study of a chemostat community, that the level of productivity determines the relative importance of competition versus predation [88].The importance of bottom-up effects is evident in the way water-column productivity (i.e. plankton) affects the diversity and growth of benthic species, including the sponge Callyspongia vaginalis. The cross-talk between feedback loops exerted through biotic top-down (predation) and bottom-up (primary productivity) effects make it difficult to distinguish the relative importance of each effect. Nevertheless, it is now accepted that negative and positive feedback shapes the characteristics of benthic communities. Abiotic factors such as water temperature, macro-scale flow velocities and solar irradiation also influence sponge biology, suggesting an ecosystem-level regulatory mechanism [89]. Cooperative interactions (a type of symbiosis) are related to bottom-up effects in the sense that there are organisms that tend to “cooperate” when bottom-up effects lead to a low abundance of resources (water, nutrients, etc.). By aggregating in large numbers, conspecifics may be able to modify the environmental conditions, via switches that involve positive feedback, to use the limited resources more efficiently [90]. Individuals may enhance their growth and food intake by settling near conspecifics. Cooperation between distinct species can lead to positive feedback by way of a local, small-scale facilitation in feeding and growth. On the other hand, as the density of the group increases, intraspecific competition for the limited resource will increase. This will lead to a large-scale negative feedback on the group, which may lead to patterning or segregation into smaller patches. The overall effect of this alternating positive and negative feedback regulation is the eventual heterogeneous pattern or patch formation, which leads to a higher population density when compared to a homogeneous population that lacks patterning [9]. Ecologists have observed that a spatially heterogeneous population has a stability that is more robust to perturbations relative to a spatially homogeneous population [91]. Additionally, experimental work has shown that some bacterial species are capable of cooperating, or competing, depending on the environmental conditions specified by the type of growth media [92]. Some examples of cooperativity between vertebrates are described in [93].Some mussel species aggregate cooperatively to increase their resistance to top-down effects, including predation and dislodgment by environmental forces [94]. By aggregating in small patches, sessile organisms are able to decrease the negative feedback exerted on them by their predators. Cooperation that leads to higher fitness via feedback mechanisms is also seen in other species, including naked mole rats [95], white-fronted bee-eaters [96], white-winged choughs [97], and bluegill sunfish [98]. Large-scale facilitation and small-scale competition has been observed in smooth cord grass [32]. Mathematical models of sessile organisms predict that interspecific cooperation will lead to a diffuse transition boundary from one species to the other along environmental gradients, via positive feedback on the survival of the cooperating species. Moreover, it has been concluded that cooperation allows certain sessile organisms to inhabit a larger region of the intertidal zone [99].
5. Discussion
- Based on the distinct hypotheses describing ecological regulatory mechanisms discussed in this review, a community can be regarded as an input-output system consisting of interacting components (populations) whose dynamics are influenced by positive and negative feedback loops. The ecological controller that acts to suppress or promote population growth can be competition, predation, bottom-up effects, cooperation or other biotic or abiotic mechanisms. Multiple controllers are usually present in any given community, each exerting its regulatory effect with a strength that varies over time, space and between trophic levels. In a short temporal scale, a disturbance may lead to brief instability due to positive feedback (unregulated growth), but in a longer timescale, the system moves slowly toward stability via negative feedback [100]. Feedback mechanisms in communities and ecosystems lead to habitable conditions, and this may explain the persistence of life on Earth [101]. If we focus our attention on fine-scale events, we might only see stochastic processes or the transient response of a system that is moving slowly toward a steady state. There may be situations, however, when the tendency of a system to stabilize and self-organize itself can only be perceived through a coarse-scale perspective. For example, positive feedback is believed to be responsible for some of the major ecological and evolutionary transitions, including increased adaptation, sympatric speciation and rapid recovery of biota after mass extinctions [102].Although the response to an ecological controlling mechanism may sometimes be slow, or the performance may not be “smooth,” the size of a population does not increase monotonically indefinitely, which indicates the presence of regulation via feedback. In addition, there may be external forces which act as regulating factors on the system, including resource availability or environmental disturbances. Mathematical models have shown that positive feedback can influence the secondary successional transient process of a community following an environmental disturbance [103]. Therefore, feedback mechanisms and changes in environmental conditions can act in concert to regulate large-scale metapopulation dynamics, even in the presence of unstable local behavior [104].In spite of experimental evidence that shows the prevalence of feedback in nature and the availability of modeling techniques and computational tools that can be used to simulate a diverse set of interactions, there are complex interactions that at first sight do not necessarily fit the traditional input-output system description. An example of the latter case is interspecific avian brood parasitism, in which a bird species lays its eggs in the nest of a different bird species, resulting in a detrimental effect on the density of the host species that cares for the eggs [105, 106]. It is sometimes difficult to view the community-level effect of parasites that live within a host, since the mechanism of action of many parasites is often unknown, and their effect on a community is sometimes not apparent, not completely understood or blurred by the presence of co-infections in the host. Discriminating the effect of parasitism on a community from the effect of other biotic factors is especially difficult when the effect of parasitism can only be observed in a timescale of years or decades and multiple ecological controllers are present. Nevertheless, experimental and computational work has contributed to our understanding of host-parasite dynamics and parasite transmission [107-111] and the role of negative feedback on parasite burden and mate selection [112].Mutualism does not fit into any of the traditional ecological paradigms of species interactions and population control. It is now know that mutualism is widespread, and the importance of feedback in this type of interaction came to be appreciated only recently. An example of a mutualistic interaction is that of the acacia plant and the ants that live on it, where the plan provides food and shelter, while the ants provide protection against herbivores and leaf pathogens [113]. Another example of a mutual symbiosis is that of corals and the zooxanthellae and endolithic algae that live in them [114]. Unfortunately, the existence of direct and indirect species interactions can sometimes make it difficult to identify the role of feedback on population dynamics, unless carefully-controlled experiments are carried out. One study elucidated the role of positive feedback in driving ant-aphid mutualism in the presence of a gall-making fly (R. salicisbrassicoides) [115]. This study concluded that an ant-aphid mutualism facilitates outbreak densities of galls via a positive feedback loop that reduces galler parasitoids. It was also observed that the aphid population growth was highest in the presence of both ants and galls. A bigger challenge for ecologists is to identify and disentangle complex feedback loops in situations where making controlled experiments is not feasible. Mathematical models of mutualistic relationships have been developed to elucidate the role of mutualism in population dynamics [116-119].Cannibalism is a selfish behavior that also does not seem to fit in with the traditional systems view of ecological interactions. Cannibalism is known to enhance the population growth and adult size and can promote density stabilization of certain species, although it can also be a destabilizing force in other populations. Progress has been made in this area through modeling efforts that incorporated the effect of cannibalism on density dynamics. This work has facilitated the discovery of counterintuitive facts about the important role of cannibalism in certain species [120-124].There are challenges to be faced when trying to study a community from a systems ecology perspective. For example, there are cases where density-dependent effects caused by behavioral aspects of a population switch a feedback loop from positive to negative, or vice versa, either gradually or sharply. For example, experiments with mussels show that aggregation of Mytilus edulis may be beneficial when small-scale interactions are considered [9], but large aggregations may have adverse effects on mussel density due to increased intraspecific competition [78, 94] and the greater probability of dislodgement due to wave energy stress. For a small population, positive feedback may dominate, but the intensity of negative feedback on population growth will increase as the density increases. Therefore, which type of feedback (positive or negative) has a greater impact on the population dynamics of an organism may depend on its density [125], which is influenced by its behavioral aspects.The spatial location of a particular organism within a group of conspecifics may determine the intensity of negative feedback, due to intraspecific competition, that the organism experiences. The benefits of aggregation may differ for sessile versus mobile species [78]. Evidence suggests that a subordinate species may benefit more from aggregation than the dominant one [126]. Moreover, it has been shown that aggregation is not a necessary condition for intense intraspecific competition. The northern short-tailed shrew, Blarina brevicauda, tends to exhibit strong intraspecific competition due to its high-energy requirements and territoriality [127, 128].Another level of input-output complexity observed in trophic cascades involves predator-detritivore-plant interaction chains, where the predatory regulatory effect is environmentally context-dependent [129] and in geographical regions where community dynamics are driven mainly by environmental stochasticity [130]. Moreover, the relative importance of resources (a bottom-up effect) and predators (a top-down effect) on the regulation of a population may depend on location [131].Changes in environmental conditions and disturbances can lead to an abrupt change in population density and structure [77], and consequently, this change will affect the feedback mechanisms acting on a system. The effect of climate change on population dynamics has been studied [132-135] and modeled [136-138], and has become a key regulatory factor that scientists will continue to monitor. Not only are communities regulated by density-dependent and density-independent feedback, but exogenous factors also play an important regulatory role. In some species, the impact of the weather on population regulation is greater than the influence of density-dependent mechanisms [139, 140]. Changes in climate may lead to direct regulation of a particular species [12, 141], or to indirect regulation through bottom-up effects, which affect primary productivity and prey recruitment, thus leading to variations in intensity of negative feedback due to predation. Although the aim of this review was to focus mainly on the effect of feedback loops on community-level dynamics, a discussion of the impact of feedback loops on terrestrial and marine communities would be incomplete if the effect of climate change at the ecosystem level were not considered. ENSO events and human impact are known to be responsible for increased fire activity in certain regions [142, 143] which, in turn, affect biogeochemical cycles [144-146]. Work on ecosystem dynamics has been carried out to assess, and project, the cascading effects of global feedback loops exerted by climate change [147-152]. Natural or man-made disturbances that change the availability of resources, such as natural or artificial fires or deforestation due to logging, can disrupt the positive feedback that reinforces the high levels of precipitation that are necessary to sustain rain forests. The end result is an ecosystem that is unable to return to its previous equilibrium point (a fully-covered forest) and instead stabilizes at a lower level of forestation. This large-scale change in vegetation leads to a trophic cascade that irreversibly affects the biodiversity of the forest. This permanent change in the level of vegetation at equilibrium is analogous to a change of a parameter value or an input that drives an electrical or mechanical system to a different basin of attraction, eventually taking the system dynamics to a different asymptotic equilibrium point. Understanding the role that feedback plays in structuring communities and ecosystems, and the role that each species plays at both levels, can help us foresee and avoid unintended consequences of human disturbances, such as insect outbreaks or the eventual extinction of a species. Large-scale vegetation-dependent communities have evolved a natural adaptation to certain periodic disturbances that do not affect the level of biomass that can potentially be supported. Since these communities depend on these disturbances, the best forestry management strategy is one that can mimic a natural disturbance as much as possible in terms of its spatial extent and frequency. Some of the main effects of disturbances on forests and strategies for pre- and post-disturbance forest management are described in [153-155]. Lastly, in systems regulated by feedback mechanisms, there are often inevitable delays in the feedback regulatory response, since the feedback mechanism responds to changes in the state after they have occurred. In ecological systems consisting of social, cooperative species, there are instances when an oscillatory or chaotic trend in population density is observed due to a delay in the regulatory feedback mechanism [156, 157]. This delay can occur if there is an uneven distribution of resources and no individual can monopolize them [158]. There could be a population explosion beyond the sustainable capacity followed by a population crash due to overcrowding or disease, which may lead to the extinction of the crashing species. This population crash is an example of a delayed, density-dependent, negative feedback effect. Social spiders, such as Aebutina binotata, are an example of a species that exhibits chaotic population dynamics [159, 160]. Mathematical models of predator-prey interactions involving a time-delay predict a strong effect of the delay on system stability, and the potential of observing oscillatory predator and prey densities [161-163].
6. Conclusions
- The rise and fall of hypotheses describing regulatory mechanisms acting on interacting species is a testament of the difficulty that ecologists face when they seek answers to questions on observed natural phenomena, and of the complex processes that are the foundation of communities and ecosystems. The integration of these hypotheses, through an application of the principles of feedback and control theory, has been instrumental in helping to identify temporal and spatial trends in community dynamics. The interdisciplinary effort by scientists has led to an understanding of communities as entities that are subject to input-output feedback control. In the light of control theory, the key components of a community can be detected, including those comprising the input, output, controller, actuator, and the process to be regulated. By working in an atmosphere of collegiality, the properly conducted experimental [34, 164] and computational work of researchers has led to the rise of systems ecology as a useful framework to study communities and ecosystems. Climate change has become the main driving force behind the current ecological transition process characterized by drastic changes in global patterns of primary productivity, biodiversity, and natural disturbance patterns. Therefore, the main focus should be on expanding the ecosystem-level studies and modelling efforts that are currently being undertaken worldwide to better predict these changes, and their effect at the community level, caused by the interconnection of multiple feedback mechanisms. A true concerted effort at the scientific, economic and political levels will allow us to prepare for these climatic changes and possibly slow down their rate of progression. Management strategies should focus on strategies to attenuate the disruptive effect of feedback processes caused by changes in natural disturbance patterns and that impact communities at distinct trophic levels. It is in this regard that a systems ecology approach, coupled with the latest technological advancements to remotely quantify the temporal and spatial extent and rate of climate change, will prove to be most valuable.
Conflict of Interest
- The author declares that there is no conflict of interest regarding the publication of this paper.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML