-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
p-ISSN: 2325-002X e-ISSN: 2325-0038
2014; 4(2): 43-50
doi:10.5923/j.zoology.20140402.02
Aflatoxicosis and Herbal Detoxification: The Effectiveness of Thyme Essence on Performance Parameters and Antibody Titers of Commercial Broilers Fed Aflatoxin B1
Manafi M., M. Hedayati, M. Yari
Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran
Correspondence to: Manafi M., Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
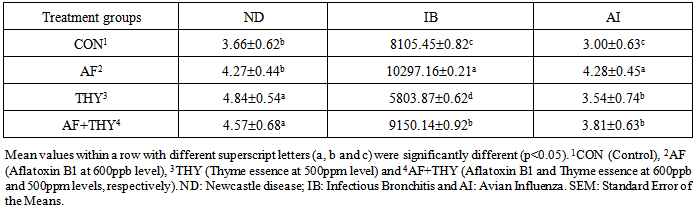
The current study is planned to investigate the effect of aflatoxin B1 (600ppb) and the ethanolic extract of Thyme (500ppm) in broiler chickens. 240 unsexed Ross broiler chicks were randomly allotted into 4 dietary treatments with three replicates and twenty chicks per replicates and reared in cage systems for 42 days. Results showed that the body weights of broilers fed aflatoxin were reduced significantly (P<0.05) and feed consumption and FCR were increased significantly (P<0.05). The inclusion of Thyme essence have showed an improved data and addition of Thyme essence to aflatoxin fed groups could partially alleviate the adverse effects of aflatoxin in the diet. The antibody titer values against Newcastle Disease, Infectious Bronchitis and Avian Influenza of broilers fed aflatoxin were significantly (P<0.05) increased and incorporation of 500ppm Thyme essence could partially restore the negative impact of aflatoxin in commercial broilers.
Keywords: Aflatoxin B1, Thyme Essence, Body Weight, Feed Consumption, FCR, Antibody titers, Broilers
Cite this paper: Manafi M., M. Hedayati, M. Yari, Aflatoxicosis and Herbal Detoxification: The Effectiveness of Thyme Essence on Performance Parameters and Antibody Titers of Commercial Broilers Fed Aflatoxin B1, Research in Zoology , Vol. 4 No. 2, 2014, pp. 43-50. doi: 10.5923/j.zoology.20140402.02.
Article Outline
1. Introduction
- Mycotoxins are secondary metabolites that are toxic to humans and animals and produced by many species of fungi. Most of them are in small molecular sizes [1]. Various investigations of mycotoxins have been carried out over the decade, mostly on emphasis on food intoxication and its prevention [2]. Mycotoxin food contamination causing mycotoxicosis in poultry and has been a serious problem, and remains so in some developing countries. Pathogenic Aspergillus spp. also produces mycotoxins which are well known for their strong and varied biological activities. For example, aflatoxin, the well-investigated mycotoxin, is known to carry the most potent carcinogenic activity as a natural product. It also carries acute toxicity to various human cells such as hepatocytes, renal cells, lung epithelioid cells, etc., as well as various immunosuppressive activities [3]. Many other mycotoxins have fairly similar activities [4]. Aflatoxins, the toxic secondary metabolites of various Aspergillus spp., are normally encountered in a wide range of tropical and subtropical feeds. These are furanocoumarin in compounds and mainly include aflatoxins B1, B2, G1, G2, and M1. Till now, much work has been done to explore the hepatotoxic, carcinogenic, and immunosuppressive effects of AFB1 [5]. When focusing on how mycotoxins play a role in food safety, attention should be given to mycotoxins that are known to be transferred from feed to food of animal origin, as this food represents a significant route of introduction for humans. Apart from their toxicological effects in affected animals, the carry-over through animal final products, such as meat, milk and eggs into the human beings food chains is an important aspect of mycotoxin impurity. FAO has estimated that up to 25% of the world’s food cereals and a higher percentage of the world’s animal feedstuffs are significantly contaminated by mycotoxins. Aflatoxin or ochratoxin residues in meat are infrequent [6]. However, it’s more common in target organs especially liver. This organ may have its lipid content increased over three fold when 20ppm aflatoxin is incorporated in broiler diet [7]. The toxicity of AF in poultry has been widely examined by determining their teratogenic [8], carcinogenic, mutagenic and growth inhibitory effects [9]. The biochemical hematological [10], immunological [7], gross and histopathology [11] toxic effects of AF have also been well documented. Preventing of mold growth and AF contamination in feed and feedstuffs is very vital but when contamination cannot be prevented, decontamination of AF is needed before using these materials. Producers, researchers and governments aim to develop effective prevention system and decontamination techniques to minimize the toxic effects of AF. Practical and cost-effective tools of detoxifying AF-contaminated feed are in great petition. Besides of the preventive management, approaches have been employed including physical, chemical and biological treatments to cleanse AF in contaminated feeds and feedstuffs. An approach to the problem has been the usage of non-nutritive and inert adsorbents in the diet to bind AF and reduce the absorption of AF from the gastrointestinal tract. Since the early 1990s, experiments with adsorbents such zeolites and aluminosilicates have proven to be successful, but high addition rates and possible potential interactions with feed nutrients are causes for concern [12, 13, 14]. Also, possible dioxin contamination may be a risk factor for using of natural clays in case of forest and trash fire near the source of them [14]. One of the approaches to overcome mycotoxicosis in poultry is using herbal products which contain essential oils. It has been well recognized that drug-metabolizing enzymes (phase-I and phase-II enzymes) and AFB1 adduct formation can be changed by natural constituents of the diet, nutrients, phytochemicals and xenobiotics [15]. Phenolic phytochemicals are thought to promote ideal health partly via their antioxidant and free radical scavenging activities thereby protecting cellular components against free radical induced damage. But due to their diverse chemical structures, they are likely to possess different antioxidant abilities [16]. Essential oils are complex compounds, and their chemical composition and concentrations of various compounds are variable [17]. Essential oils basically consist of two classes of compounds, the terpenes and phenylpropenes, depending on the number of 5-carbon building blocks. The exact anti-microbial mechanism of essential oils is poorly understood. However, it has been suggested that their lipophilic property [18] and chemical structure [19] can play a role. It was suggested that terpenoids and phenylpropenes can penetrate the membranes of the bacteria and reach the inner part of the cell because of their lipophilicity [20]. Moreover, structural properties, such as the presence of the functional groups [19] and aromaticity [21] are also responsible for the antibacterial activity of essential oils. Thyme (Thymus vulgaris), a member of Lamiaceae family, with the main components of phenols, thymol (40%) and carvacrol (15%). This herb is also used traditionally for diseases and its beneficial value has been reported in poultry [22]. Also, yogurt, due to its probiotic potential, can be used instead of commercial antibiotics, too. The main probioticts in yogurt are lactic acid bacteria, and it has been reported, that L. acidophilus can absorb cholesterol from in vitro system. In addition, it can increase the protein digestibility and availability of minerals, viz. Cu, Mn, Ca, Fe, P for its host [23]. Thyme has been commonly used in foods mainly for the flavor, aroma and preservation and also in traditional medicine since the ancient Greeks, Egyptians and Romans. The leafy parts of thyme belonging to the Lamiaceae family are often added to meat, fish and food products and also used as herbal therapeutic foodstuffs. Evidence based research hypothesize that thyme possesses numerous biological activities including antispasmodic, antimicrobial, antioxidant and antifungal. Moreover, thyme extract possess antioxidant activity and hinders lipid peroxide formation [24]. The supplementation of poultry diets with essential oils led to enhanced weight gain, improved carcass quality and reduced mortality rates. The aims of the current study were to determine the activity of the ethanolic extract of thyme in vivo and to evaluate the protective effects of the extract on performance and antibody titers of broiler chickens fed AFs contaminated diet.
2. Materials and Methods
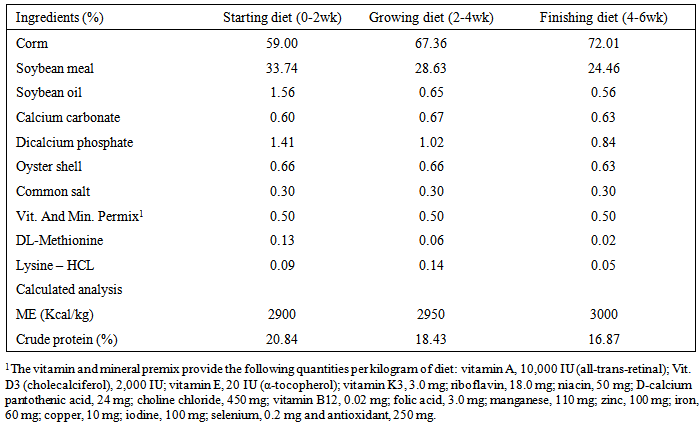
- This experiment was planned and carried out in the Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran with objective of evaluating the performance and immune response of broilers fed with aflatoxin B1 and Thyme essence. Experimental design, housing, management and test diet240 day-old unsexed Ross 308 strain of broiler chicks were wing banded, weighed and randomly spread in a completely randomized experimental design with four treatments and three replications of twenty chicks in each. Each replicate group of chicks was housed in an independent pen, conventional deep litter house. Chicks in all the replicates were kept up to six weeks of age under uniform standard conditions. Brooding was done till three weeks of age. Each pen was fitted with an automatic bell type drinker and a hanging tubular feeder. Chicks were provided ad libitum feed and water throughout the study. Feeding of test diets commenced at first day of age and continued till the termination of experiment at six weeks of age. The temperature was maintained at 30±1°C in the first week and reduced by 2.5°C per week to 21°C. From day one until day 4, the lighting schedule was 24 hour. At days 14-42 the dark time was gradually increased to 4 hour. Diets were prepared to meet the nutrient requirements of commercial broilers during the starter (0-2 weeks), grower (2-4wks) and finisher (4-6 weeks) periods. The composition of diets was adopted from NRC, [25] and is presented in Table 1. The basal diet was formulated using commonly available feed ingredients which were screened for AF prior to the formulation of diets. The Aflatoxin B1 was procured from Sigma Aldrich, USA and diluted to reach to the required level of administration. The experimental diets were prepared by adding required quantity of aflatoxin to arrive at the levels of 0 and 600ppb of AFB1. Diets were prepared without addition of aflatoxin and Thyme essence as Control (group 1); 600 ppb Aflatoxin B1 (group 2); 500ppm of Thyme essence (group 3) and 600ppb Aflatoxin B1 + 500 ppm of Thyme essence (group 4). The ethanolic extraction of Thyme was prepared as per the instruction given below:Plant materialCollective samples of the aerial parts from Thymus capitatus growing wild in Khoramabad region within Lorestan province in Iran were collected during the Sept. 2013. Collected plant materials were dried in the shade, and the plant leaves were separated from the stem, and grounded in a grinder to small particles.
|
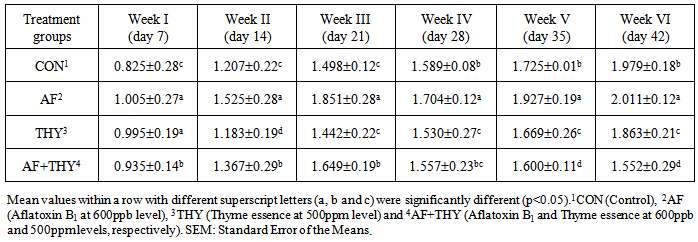
3. Results and Discussion
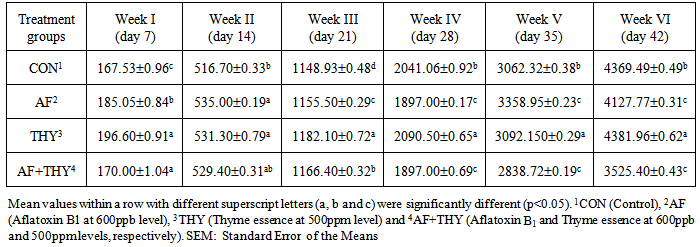
- The effects of aflatoxin and Thyme essence on body weight (g): The results of dietary treatments on weekly body weight of broiler chicks are shown in Table 2. Results showed that the weight of day-old chicks were uniform and in a similar range. At day7, the AF fed group have shown a significantly (P<0.05) lower body weight, compared with control group. The group fed Thyme extract have shown a non-significant changes compared with control group. In AF+THY group, the reduced body weight of chicks due to presence of AF could not be reached up to the control level. At day 14, AF fed group could significantly (P<0.05) reduce the body weight, compared with control group. In group fed Thyme essence, the maximum body weight were found and in group fed AF+THY, the minimum BW has been found. This indicates that all 3 groups of AF, THY and AF+THY have shown a significant changes in BW, when compared with control group. At day 21 and day 28, the same trend of day 14 have been found in all treatment groups. At day 28, the body weight is found to be higher in THY fed group, followed by control, AF and AF+THY fed groups. At this day, again the toxic effects of AF could not be reached by Thyme essence administration in broiler diet. At the end of trial, the control group showed a weigh of 2207.93g BW, whereas AF fed group is found 2052.60g, which indicates a significant (P<0.05) reduce in BW of broilers. Similar to other weeks, BW of group fed Thyme extract found to the higher among all treatments, compared with control group and in AF+THY fed group, the BW could not reach to the control group. This means that Thyme extract has no ability to significantly (P<0.05) reduce the adverse effects of aflatoxin in broiler diet.
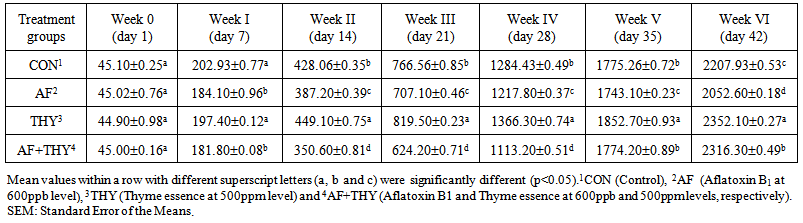 | Table 2. Body weight (g) of chicks fed Aflatoxin B1 and Thyme essence (Mean±SE) |
|
|
|
4. Conclusions
- Based on the founded data in this trial and available reports, it could be concluded that the addition of aflatoxin in broiler diet can have a negative impact on the performance and immune response of broilers and Thyme essence can be used as natural non-antibiotic feed additive on broilers. Nevertheless, there is scarcity in the evidence of its beneficial effects in nutrient digestibility and gut function of broilers fed with aflatoxin and Thyme essence.
ACKNOWLEDGEMENTS
- This study was funded by Directorate of Research, Malayer University, Malayer, Iran.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML