-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
p-ISSN: 2325-002X e-ISSN: 2325-0038
2014; 4(1): 13-19
doi:10.5923/j.zoology.20140401.03
Application of Rosemary (Rosmarinus officinalis L.) Essence on Chicks Fed Aflatoxin B1: Impacts on Internal Organ Weights, Biochemical Traits and Mortality
Manafi M. , M. Hedayati , M. Yari
Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran
Correspondence to: Manafi M. , Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
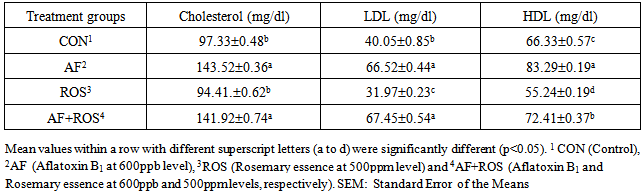
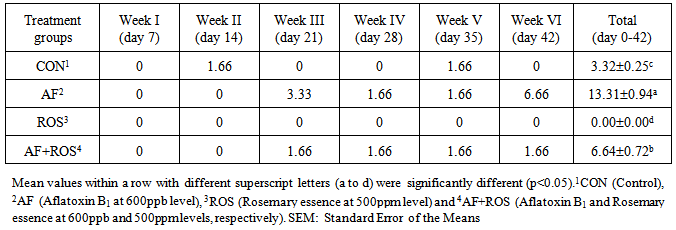
Aflatoxins (AF) are a major issue encountered in poultry farms, leading to much economic losses. The current trail is planned to determine the effects of AF (600ppb) and Rosemary essence (ROS) (500ppm), singly or in combination, on the visceral organ weights, some blood biochemical parameters and mortality percentage of broilers. A total of 240 unsexed Ross 308, were randomly allotted into 4 treatments with 3 replicates having 20 chicks each (Control; AF; ROS and AF+ROS). The broilers fed AF contaminated diet from beginning and showed characteristic effects of aflatoxicosis. The weight of spleen, and pancreas decreased and bursa and liver weights increased due to ingestion of AF. Serum levels of cholesterol, LDL and HDL and mortality is increased in aflatoxin fed broilers. The Rosemary could partially restore the adverse effects of AF to some extent and when alone added into the diet and showed good effects on reducing cholesterol, HDL, LDL and reduction number of dead birds, due to presence of essential oils and antioxidants activity in its content.
Keywords: Aflatoxin B1, Rosemary Essence, Visceral Organs, Cholesterol, LDL, HDL, Broilers
Cite this paper: Manafi M. , M. Hedayati , M. Yari , Application of Rosemary (Rosmarinus officinalis L.) Essence on Chicks Fed Aflatoxin B1: Impacts on Internal Organ Weights, Biochemical Traits and Mortality, Research in Zoology , Vol. 4 No. 1, 2014, pp. 13-19. doi: 10.5923/j.zoology.20140401.03.
Article Outline
1. Introduction
- Several species of fungi producing toxin compounds are found relatively commonly in cereal grains, either at harvest or during storage when these crops are harvested under unfavorable weather conditions or improper storage. Mycotoxins are low molecular compounds of fungal metabolites capable of causing toxic responses in various organisms. In recent years, it has become demonstrated that different animal species and particular poultry can be further affected by mycotoxins produced by specific fungal contaminates. Mycotoxin can enter the system of an animal by ingestion, inhalation or direct skin contact. Very little amounts of those toxins can cause significant health problems. Aflatoxins (AF) are a group of chemicals produced mainly by Aspergillus, primarily by Aspergillus flavus and Aspergillus parasiticus (Manafi et al., 2009). The aflatoxin B1 (AFB1) is the most prevalent toxin in cereal used in animal and poultry feeds (Santurio, 2000). The mutagenic and carcinogenic effects of AF are well known and the liver is the main target organ of this mycotoxin. Metabolic changes associated with liver damage, decreased activity of digestive enzymes, immune suppression (Osweiler, 1990) and more recently, changes in gene expression of liver enzymes and changes in intestinal morphology and function have also been reported. The deleterious effects of AF vary according to the dose, exposure time, sex, and age of the animal (Manafi and Khosravinia, 2013). The primary effects of aflatoxicosis in birds may be used for the clinical judgment of the disease. One of the most important signs of aflatoxin toxicity in broilers is the change in internal organs. Liver, spleen and kidney increase in size (Osweiler, 1990), while the bursa of Fabricius and the thymus decrease (Sur and Celik, 2003). Giambrone et al. (1978) reported that aflatoxins in birds have toxic effects on immunological responses, causing the inhibition of protein synthesis and consequently a decrease in the production of antibodies. These toxic effects are also related to decreased vaccine responses and occurrence of unspecified diseases (Corrier, 1991). Therefore, there is wide interest in the use of biological products to decrease mycotoxin availability. One of the alternatives is the use of herbal adsorbents, which bind to mycotoxins and prevent their absorption by the gastrointestinal tract, making them inert to animals (Huwig et al., 2001). Inorganic and biological binders have been investigated in studies to control the bioavailability of mycotoxins. Glucan-based binders, produced from carbohydrates of the cell wall of some species of yeast, have been well studied (Kassie et al., 2002). One of the recently attracted tools to hinder the adverse effects of AF is the use of herbal and medicinal products. Among the various medicinal and culinary herbs, some phenolics are ubiquitous compounds found in all plants as secondary metabolites; these include simple phenols, hydroxybenzoic acid and cinnamic acid derivatives, flavonoids, coumarins and tannins (Naczk and Shahidi, 2004). They may be used for the production of raw materials or preparations containing phytochemicals with significant antioxidant capacities and health benefits (Naczk and Shahidi, 2004). Crude extracts of fruits, herbs, vegetables, cereals and other plant materials are rich in phenolics and are increasingly of interest to the food industry because they retard the oxidative degradation of lipids and thereby improve the quality and nutritional value of food (Protestos et al., 2006). Rosemary (Rosmarinus officinalis L.) is a well-known aromatic plant used all around the world for different medicinal purposes. Recent research has shown that rosemary extracts have a variety of pharmacological activities, such as antimicrobial, antioxidant, cognition- improving, cancer chemoprevention and DNA-protective effects (Tsukamoto et al., 1995). Natural antioxidants are applied to foods in two ways, either direct use or by using their extracts. Rosemary and origanum are medicinal plants that have strong antioxidant activity. The various extracts and essential oils from rosemary and origanum were previously used as antioxidants in different models (Yanishlieva et al., 2006). Rosemary leaves contain up to 2 % essential oil, about 8 % tannins, ursolic acid, flavonol compounds, alkaloids, vitamins, minerals and other biologically active substances. Tinctures and infusions from rosemary leaves have been applied in traditional medicine for treating rheumatism, gout, neurosis, eczemas, decubitus wounds, mouth inflammation and other health problems. Rosemary is one of the most effective spices widely used in food processing as well it is the only spice commercially available for use as an antioxidant in Europe and the United States. There are a limited number of studies about the direct application of natural antioxidants. Antioxidant properties of rosemary have been well documented (Yanishlieva et al., 2006). It is considered as lipid antioxidant, metal chelator and super oxide radical’s scavenger (Carrillo and Tena, 2005). Many different solvents, as well as extraction techniques have been used for the isolation of the antioxidative compounds (Szumny et al., 2010). However, up to our knowledge, there is no available information concerning usage of rosemary essential oil or its extracts on the inhibition of mycotoxins.
2. Materials and Methods
- This experiment was planned and carried out in the Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran with objective of evaluating the internal organ weights, biochemical and mortality of broilers fed with aflatoxin B1 and Rosemary essence.
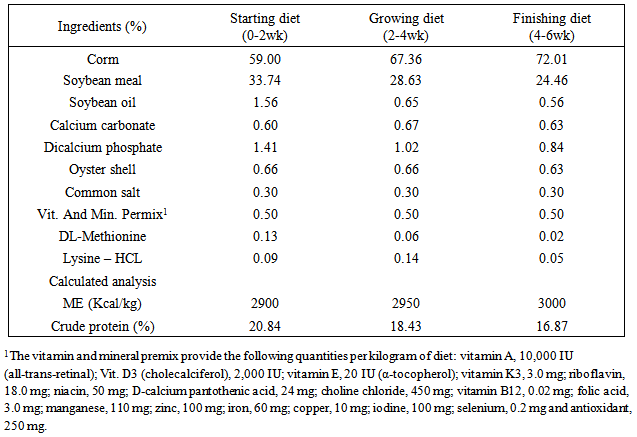
2.1. Experimental Design, Housing, Management and Test Diet
- 240 day-old unsexed Ross 308 strain of broiler chicks were wing banded, weighed and randomly spread in a completely randomized experimental design with four treatments and three replications of twenty chicks in each. Each replicate group of chicks was housed in an independent pen, conventional deep litter house. Chicks in all the replicates were kept up to six weeks of age under uniform standard conditions. Brooding was done till three weeks of age. Each pen was fitted with an automatic bell type drinker and a hanging tubular feeder. Chicks were provided ad libitum feed and water throughout the study. Feeding of test diets commenced at first day of age and continued till the termination of experiment at six weeks of age. The temperature was maintained at 30±1°C in the first week and reduced by 2.5°C per week to 21°C. From day one until day 4, the lighting schedule was 24 hour. At days 14-42 the dark time was gradually increased to 4 hour. Diets were prepared to meet the nutrient requirements of commercial broilers during the starter (0-2wks), grower (2-4wks) and finisher (4-6 wks) periods. The composition of diets was adopted from NRC, (1994) and is presented in Table 1. Diets were prepared without addition of aflatoxin and Rosemary essence as Control (group 1); 600 ppb Aflatoxin B1 (group 2); 500ppm of Rosemary essence (group 3) and 600ppb Aflatoxin B1+500 ppm of Rosemary essence (group 4). The Aflatoxin B1 was procured from Sigmal Aldrich, USA and diluted to reach to the required level of administration. The ethanolic extraction of Thyme was prepared as per the instruction given below:Plant materialCollective samples of the aerial parts from Thymus capitatus growing wild in Khoramabad region within Lorestan province in Iran were collected during the Sept. 2013. Collected plant materials were dried in the shade, and the plant leaves were separated from the stem, and grounded in a grinder to small particles.ExtractionMaceration ExtractionThe powder of T. capitatus (leaves and stems) young flowers was macerated with 70% ethanol (1:20, w/v) at room temperature for 2 days and filtered through a Whatman no.1 filter paper. Other portions of the solvent were added to the marc and the extraction was repeated until the last extract was colorless. The extracts were combined and concentrated under reduced pressure at 65ºC, 15 rpm and 90 minutes, using a rotary vacuum evaporator. The crude extract was then evaporated on a boiling water bath (HANSHIN Scientific Co, South Korea) until a constant weight was obtained to afford the maceration extract.Steam distillation ExtractionAir-dried of T. capitatus leaves were submitted for 3 h to steam distillation using a Clevenger apparatus to produce the essential oil in a yield of 5.6% (w/w). Oil was dried over anhydrous sodium sulphate and after filtration, stored at 4°C until used.
|
2.2. Vaccination Schedule
- The local office of Iranian Veterinary Organization has proposed the required vaccination which is modulated by the veterinarian of Department of Animal Science, Malayer University, based on the titers obtained from blood samples of chicks at different ages, as below:Vaccination for Newcastle Disease (ND) virus happened three times: first spray at one days old of chicken in breeder farm, second on the 13th day as B1, BRONHOPEST B1 SPF (VETERINA, GENERA®), Zagreb, Croatia) and (CEVA SANTE ANIMALE, Libourne, France) in drinking water and their booster on 20th day as clone-30 (HIPRAVIAR® CLON, Amer, Spain) through drinking water. Vaccination against Bronchitis virus happened in two times as the following: first spray at commencement of the experiment and it’s booster in drinking water on the 10th day, both as H-120 (CEVA SANTE ANIMALE, Libourne, France). Vaccination against Infectious Bronchitis (IB) virus happened in two times: first on day 16 and the second on the 23th day, both as Gambo-l (CEVA SANTE ANIMALE, Libourne, France) in drinking water. The sera were applied to HI test in 28 days, to determine Ab to NDV. In titers lower than 5, the booster B1, BRONHOPEST B1 SPF (VETERINA, GENERA®), Zagreb, Croatia) was administrated in drinking water for broilers.
2.3. Studied Parameters
- Visceral Organ WeightsUpon obtaining the permission of Ethical Committee of the University, at the end of the trial, six birds from each replicate which were closed to average weight of each replicate were sacrificed by cutting the jugular vein method and blood samples were individually collected in 10-mL heparinized tubes and stored on ice cubes for further hematology analysis. The visceral were then opened and the thymus, spleen, bursa of Fabricius, liver, kidney and pancreas detached and weighed on digital top pan electronic balance (0.1g accuracy) and the later three weighed on manopan balance (1mg accuracy). The weights were adjusted to one kg live weight (g/kg BW) and treatment wise means were calculated.
2.4. Biochemical Parameters
- The collected blood samples were analyzed for cholesterol, LDL and HDL using automatic analyzer (Boehringer Mannheim Hitachi 704 automatic analyzer, Japan). The methodology and the set of reagents used in respect of each parameter were as per the recommendations of the manufacturer of the analyzer system. Data are presented as means of each treatment.
2.5. Mortality
- The number of dead birds in each replicate was recorded to calculate the rate of mortality. The dead birds were subjected to postmortem examination to identify the cause of death. The weekly per cent mortality up to 6th week was computed.
2.6. Statistical Analysis
- The total experimental data were statistically analyzed using the General Linear Model procedure of the Statistical Analysis System (SAS®) software (SAS Institute, USA, 2000). Overall data were analyzed using one way ANOVA test. Duncan multiple range test at 0.05 probability level was employed for comparison of the means (Duncan, 1955).
3. Results and Discussion
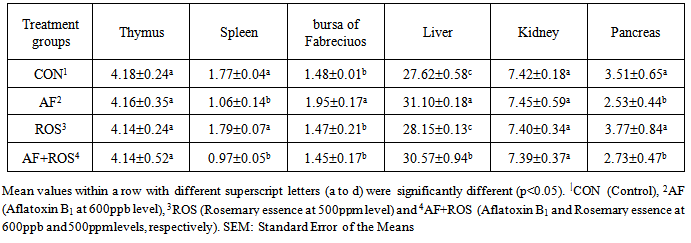
- The effects of aflatoxin and Rosemary essence on visceral organ weights (g/kg live weight): The results of dietary treatments on organ weights of broilers fed AF and Rosemary and their combinations on broilers at 42 days of age are shown in Table 2. Results revealed no sign of any significant changes in thymus weights of broilers fed AF or Rosemary essence in any treatment groups. Spleen weight is decreased significantly (P<0.05) in group fed AF, when compared with control group. Addition of ROS into the diet had shown no significant changes compared with CON. Addition of ROS into AF (group 4) has showed no improvement in the decreased spleen weight caused by AF. The weight of bursa of Fabreciuos in broilers fed AF alone fed group have showed a significant (P<0.05) increase when compared with control group and addition of ROS into the normal diet (group 3) and in addition with AF (group 4) had no significant differences with control group. The weight of liver - the most important internal organ - has been showed that AF caused a significant (P<0.05) increase in liver weight when compared with control group. Broilers fed in group 3 (Rosemary essence) have shown no significant changes with control group and in group AF+ROS, liver weight is decreased in comparison with CON which indicates that ROS has no effect on compensating the increased liver weight due to aflatoxin ingestion. Kidney weight had remained unchanged and showed a non-significant difference in all treatment groups. In case of pancreas, the weight of this internal organ is decreased significantly (P<0.05) due to presence of aflatoxin in the diet, which addition of ROS into the AF diet could not restore this increased weight. However, the ROS alone fed group showed no changes in pancreas weight, when compared with control group.
|
|
|
4. Conclusions
- Results obtained from this study concludes that besides the adverse and negative effects of aflatoxin B1 in the diet on visceral organ weight, some biochemical parameters and mortality of broilers, combining Rosemary essence as natural non- antibiotic growth promoter feed additive can rule its beneficial effects to some extent. However, there is insufficiency in the marks of its beneficial effects in nutrient digestibility and gut function of broilers.
ACKNOWLEDGEMENTS
- This study was funded by Directorate of Research, Malayer University, Malayer, Iran.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML