-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
p-ISSN: 2325-002X e-ISSN: 2325-0038
2014; 4(1): 8-12
doi:10.5923/j.zoology.20140401.02
Acute Effects of NaCl and Na2SO4 on Daphnia menucoensis Paggi, 1996 and Moina eugeniae Olivier, 1954 (Crustacea, Cladocera)
Cabrera Gabriela, Vignatti Alicia, Echaniz Santiago
Facultad de Ciencias Exactas y Naturales. Universidad Nacional de La Pampa. Avda. Uruguay 151. Código Postal 6300. Santa Rosa, La Pampa
Correspondence to: Cabrera Gabriela, Facultad de Ciencias Exactas y Naturales. Universidad Nacional de La Pampa. Avda. Uruguay 151. Código Postal 6300. Santa Rosa, La Pampa.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Despite the fact that halophilic cladocerans are relatively scarce, the neotropical endemic species Daphnia menucoensis and Moina eugeniae are frequent in temporary saline lakes of the semiarid centre and the north of Patagonia (Argentina). Although aspects of its biology in natural conditions are already known, there is a lack of ecophysiological information to help explain its geographical and temporary distribution. Considering that Cl- and SO42- are the predominant anions in La Pampa lakes, the aim of this study was to determine the effects of increasing NaCl and Na2SO4 concentration on neonates of both species, using acute bioassays, and to compare the results with previous information. The medium was prepared using demineralized water and different concentrations of pure salts of NaCl and Na2SO4 of analytical quality as well as a mixture of both salts; six concentrations for D. menucoensis (0.5; 1; 5; 10; 15 and 20 g.L-1) and four for M. eugeniae (1; 5; 10 and 15 g.L-1). Four replicates were performed, with five neonates per treatment. The bioassays took 48 h, without food or renewal of the medium. The survival of D. menucoensis was virtually zero in treatments with 15 or 20 g.L-1, but survival was higher at lower concentrations. Survival in treatments with salt mixtures differed, but was much lower at 10 g.L-1. The survival of M. eugeniae was zero in all treatments of 10 g.L-1. In previous bioassays with salts obtained from the natural environment, the tolerance of the neonates of both species was greater. D. menucoensis survived up to 24 g.L-1, while M. eugeniae survived up to 31 g.L-1. The lower tolerance observed in this study might be because analytical-grade salts were used. The absence of other electrolytes that are necessary to maintain a relatively stable internal environment of the neonates might explain the limited survival at concentrations higher than 10 g.L-1.
Keywords: Bioassays, Daphnia menucoensis, Moina eugeniae, Halophyllic cladocerans, Salinity tolerance
Cite this paper: Cabrera Gabriela, Vignatti Alicia, Echaniz Santiago, Acute Effects of NaCl and Na2SO4 on Daphnia menucoensis Paggi, 1996 and Moina eugeniae Olivier, 1954 (Crustacea, Cladocera), Research in Zoology , Vol. 4 No. 1, 2014, pp. 8-12. doi: 10.5923/j.zoology.20140401.02.
1. Introduction
- Many temporary shallow lakes of relatively high salinity exist in Argentina, especially in the semi-arid central region and in northern Patagonia. These ecosystems are characterized by associations of cladocerans and copepods, with some different elements to ecosystems in other continents, due to the presence of many endemic species with their own biogeographical patterns[1, 2, 3, 4, 5, 6, 7]. Since salinity is one of the dimensions of the ecological niche that strongly affects the distribution of organisms, the Cladocera are relatively scarce in saline lakes[8, 9]. However, the halophilous species Daphnia menucoensis and Moina eugeniae are frequent and have a wide distribution in aquatic environments of central Argentina[1, 10, 11]. Daphnia species are rare in saline ecosystems[12, 13, 14] because it was originated in freshwater where it has its major distribution[15, 16]. The frequent presence of D. menucoensis distinguishes between high salinity ecosystems in Argentina and those at different latitudes[17, 18]. Currently, experimental information exists concerning the negative effects of salinity on different population parameters of some zooplanktonic cladoceran species of other latitudes, such as Daphnia magna[9, 19, 20], Daphnia carinata[21], Moina hutchinsoni[22], Alona rectangula, Ceriodaphnia dubia, Daphnia pulex, Moina macrocopa and Simocephalus vetulus[23], Daphnia exilis[24] and Daphniopsis australis[25]. However, although some aspects of the biology in natural conditions are already known for the two Argentinean species[10, 11, 26], bioassays to determine salinity tolerance have only been performed recently[27]. Therefore, there is lack of information concerning other relevant ecophysiological aspects that explain their geographical and temporal distribution, such as the effects of different concentrations of Cl- and SO42- anions. This is because in most hypersaline and mesosaline lakes in La Pampa, where D. menucoensis and M. eugeniae occur, NaCl and Na2SO4 predominate. It was also determined that the ratio between Cl- and SO42- is very variable[28]. Therefore, the objective of this study was to determine the effects of increasing concentrations of NaCl, Na2SO4 and a mixture of both salts in the same ratio, on D. menucoensis and M. eugeniae neonates using laboratory bioassays, and to compare the results with information obtained in previous studies.
2. Materials and Method
- Acute bioassays were carried out, considering immobility or death of neonates as indicators of the effect of the anions.To obtain organisms to carry out the bioassays, sediment from the dried bottom of a saline lake was collected in La Pampa province. D. menucoensis and M. eugeniae generally integrate the zooplankton of this lake during the hydrophases. The sediment was placed in a 300 L water tank outdoors. The physiochemical characteristics of the water were similar to those of the original lake when the species were recorded. Once both populations had developed and established from ephippia of the ‘egg bank’, parthenogenetic females were removed and were separately acclimatised in the laboratory in two 20 L aquaria for 90 days. Neonates, (younger than 24 h), which were born from the selected females, were used for the assays.The experimental medium was prepared using demineralized water to which reagents of analytical grade were added. In bioassays with D. menucoensis, three series with six concentrations: 0.5; 1; 5; 10; 15 and 20 g.L-1 of NaCl, Na2SO4 or mixtures of both salts in the same ratio were prepared (a total of 18 treatments). For M. eugeniae, three series with four concentrations: 1; 5; 10 or 15 g.L-1 NaCl, Na2SO4 and a mixture of both salts were prepared (a total of 12 treatments). Four replicates per treatment were perfor- med, comprising five neonates each. The bioassays were carried out in 20 mL glass tubes and lasted for 48 h without food or renewal of the medium. Neonates were exposed to a photoperiod of 8:16 h darkness:light. The lighting was provided by two 18 Watt fluorescent tubes and the temperature remained constant at 22 ± 1°C. For D. menucoensis, the observations were performed every 12 h and for Moina eugeniae, every 24 h.To determine differences between the treatments, the Kruskal Wallis non-parametric test[29, 30] and the Mann-Whitney test[31] were performed.
3. Results
- Daphnia menucoensisIn NaCl bioassays, neonate survival at 48 h differed significantly (H = 21.36; p = 0.0079) and was almost complete at 10 g.L-1 but was almost zero at the two higher concentrations, since only one individual survived at 15 g.L-1 (Figure 1A). Although the number of survivors gradually decreased at 15 g.L-1, the decrease was abrupt in the 20 g.L-1 treatment and no survivors were observed after 12 h. Neonate survival in treatments with Na2SO4 was also significantly different (H = 21.77; p = 0.0036) (Figure 1B). As for NaCl, survival was relatively similar for treatments up to 10 g.L-1 Na2SO4, although it was higher than that at the two higher concentrations. In contrast to the NaCl treatment, although survival was zero after 48 h at the highest concentration, the mortality did not decrease abruptly (Figure 1B).
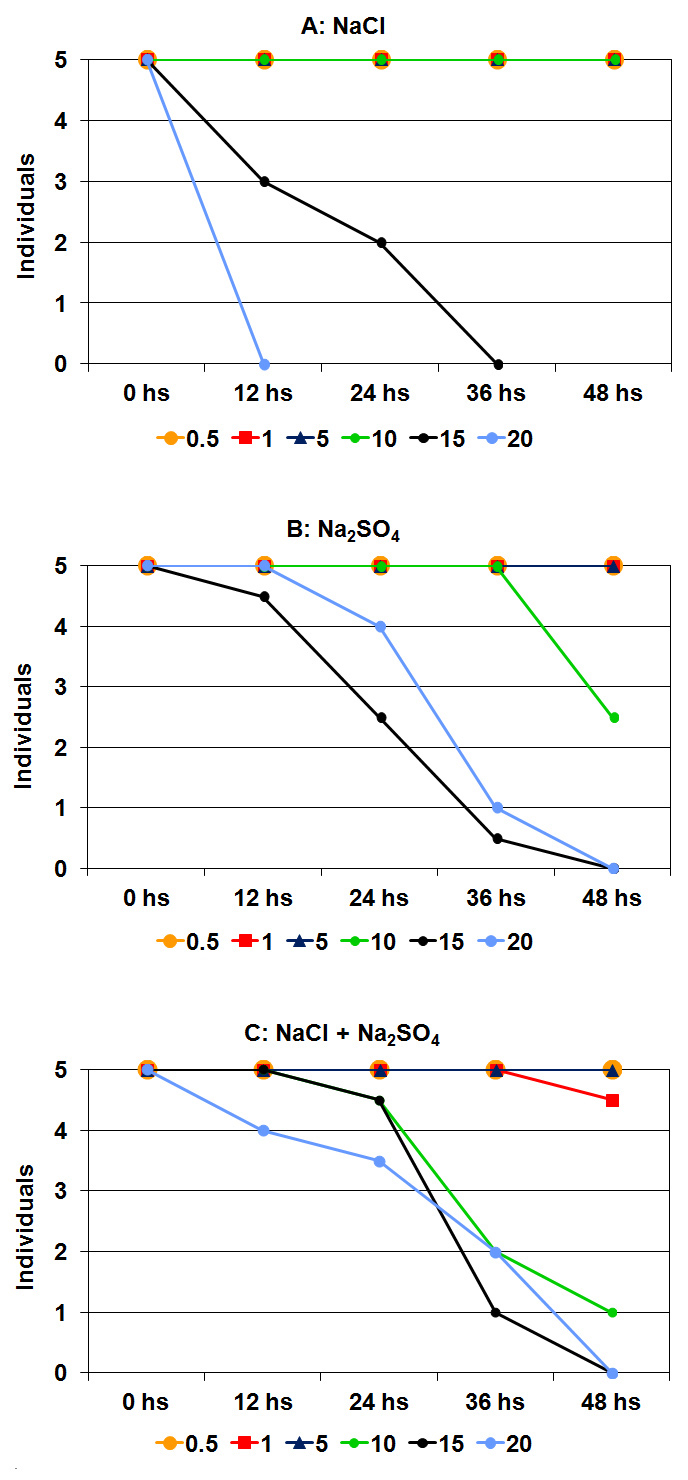 | Figure 1. Results of bioassays carried out with Daphnia menucoensis at different concentrations of individual salts and mixtures. The me-dian of the four replicates is shown |
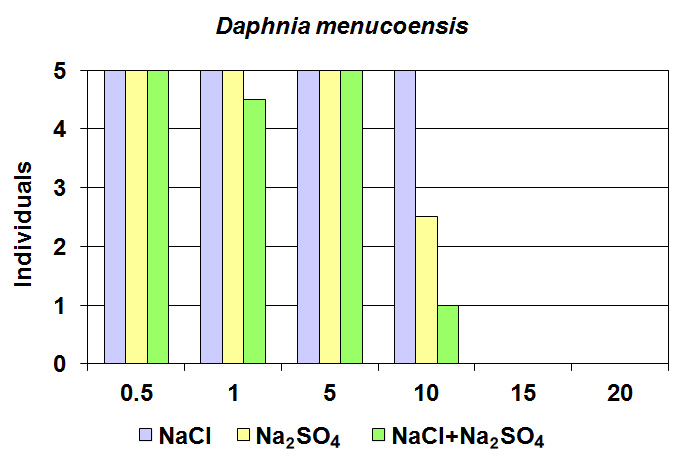 | Figure 2. Comparison of bioassays results for Daphnia menucoensis at different salt concentrations. The bars indicate the medians |
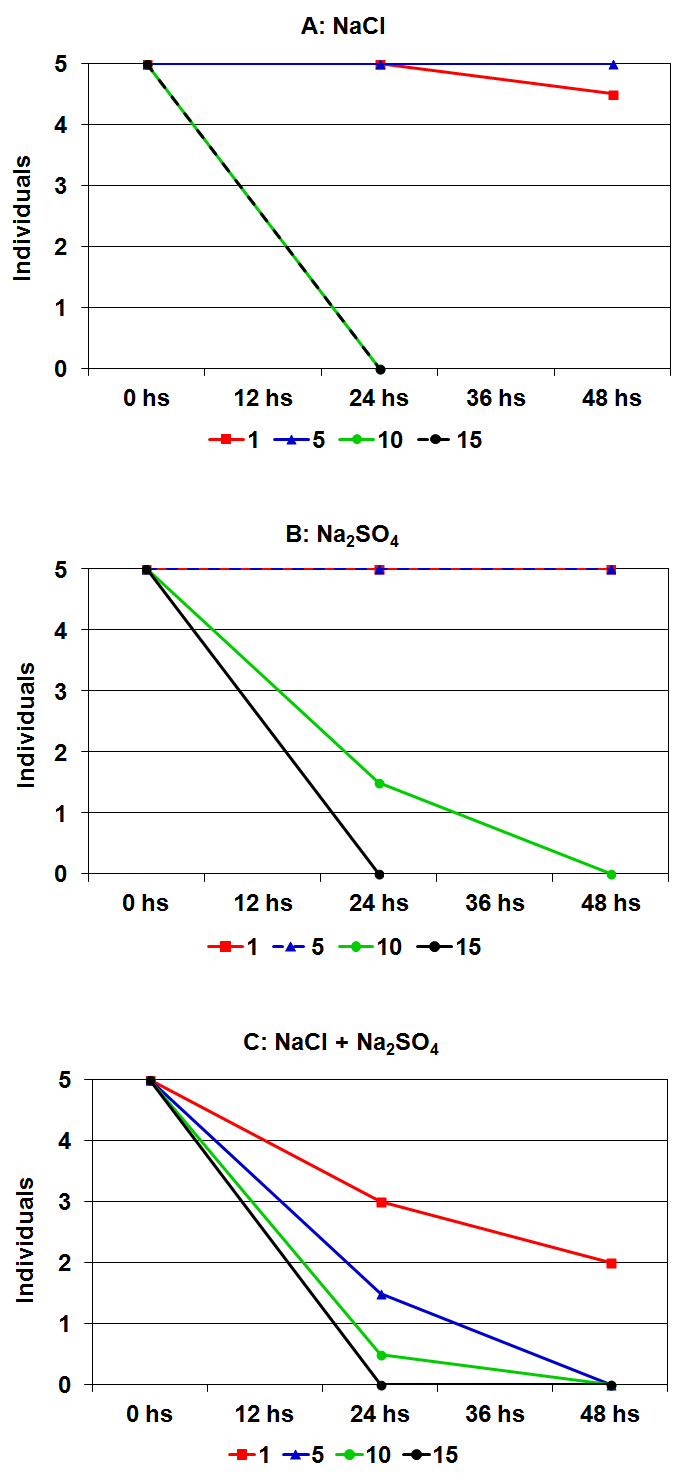 | Figure 3. Results of bioassays carried out with Moina eugeniae and different salt concentrations. The median of four replicates is shown |
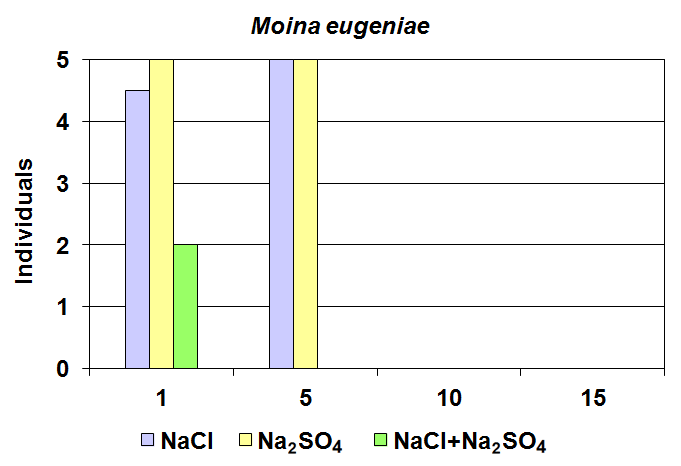 | Figure 4. Comparison of bioassays carried out with Moina eugeniae at different salt concentrations. The bars indicate the medians |
4. Discussion
- The bioassays in this study confirmed that the neonate survival of D. menucoensis and M. eugeniae was very limited after 48 h. The data were different to those of Vignatti et al. 2012[27], in the unique laboratory study done to date to determine the salinity tolerance in neonates of both species. These authors using a similar bioassay, but using salts obtained from the natural environment. In that case, precipitated salts were collected and sterilized during the drying of a temporary lake in which the presence of both species was recorded during the previous hydrophase. In those bioassays, D. menucoensis survival at a concentration up to 24 g.L-1 was observed, and the difference was even more apparent for M. eugeniae, whose neonates survived up to 31 g.L-1. On the other hand, the low tolerance of both species to relatively high salt concentrations in the present study, contrasts with results obtained in field studies. In a study that included ten shallow lakes in La Pampa province, D. menucoensis was recorded in a salinity range between 9.64 and 29.70 g.L-1, whereas M. eugeniae was found between 11.20 and 35.82 g.L-1[28].However, similar to results of bioassays carried out by Vignatti et al. 2012[27], this study showed that both species tolerate a much lower concentration of solutes than in nature. Neonates of both species survived at concentrations lower than 1 g.L-1 (or ever lower for D. menucoensis), whereas in natural conditions in water bodies from La Pampa, individuals were only recorded when the salinity was above 7 g.L-1 (M. eugeniae) or 5 g.L-1 (D. menucoensis)[10, 11, 26, 32, 33, 34]. The absence of both cladocerans in low salinity water bodies might be due to biotic interactions that might be more important in environments with low stress than physiochemical factors in community structuring[35]. Although they are adapted to low salinity, M. eugeniae and D. menucoensis would not be successful in competition for resources with other species, such as Moina micrura and Daphnia spinulata, two frequent cladocerans in water bodies from La Pampa province, which are present at salinities lower than 5–7 g.L-1[27, 32].A discussion has existed concerning the convenience of developing bioassays with pure salts as a salinity source, especially NaCl, with organisms that come from water bodies with different ionic composition[36]. The limitations of the results would be higher especially when dealing with organisms whose origin is not marine[23], like the cladocerans[7, 37]. In comparison with previous bioassays, the lower tolerance range determined in this study might be because the salts used for medium preparation were of analytical grade. Therefore, the absence of other necessary electrolytes to maintain the stability of the internal medium of the neonates, might explain the limited survival at concentrations higher than 10 g.L-1 NaCl or Na2SO4 or the mixture of both salts. The results also show that the information obtained from using pure salts does not necessarily depict the salinity ranges that the studied species tolerate in natural conditions.
ACKNOWLEDGEMENTS
- To the Facultad de Ciencias Exactas y Naturales de la Universidad Nacional de La Pampa for the financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML