-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
p-ISSN: 2325-002X e-ISSN: 2325-0038
2014; 4(1): 1-7
doi:10.5923/j.zoology.20140401.01
Wuchereria bancrofti Infection Rates in Humans (Vertebrata: Hominidae) and Mosquitoes (Diptera: Culicidae), Akwa Ibom State, Nigeria
Veronica I. Itina1, 2, M. Aline E. Noutcha1, Samuel N. Okiwelu1
1Department of Animal and Environmental Biology, University of Port Harcourt, Nigeria
2Current Address: Ministry of Health, Uyo, Akwa Ibom State, Nigeria
Correspondence to: Samuel N. Okiwelu, Department of Animal and Environmental Biology, University of Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The dearth of information on the epidemiology of Bancroftian filariasis in Akwa Ibom State, Nigeria, necessitated a 2-year study on filarial infection rates in humans and mosquitoes in rural (Abat, Odot, Nung-Udoe, and Oko-Ita) and urban (Ikot Ebok, Edisiong, Iba-Oku and Ibiakpan) communities across ecovegetational zones (Mangrove swamp forest, Lowland rainforest, Freshwater swamp forest, Moist savanna woodland). The spray catch method was used for mosquito collection. They were age-graded and filarial species identified by standard keys. Blood was obtained from consenting outpatients at hospitals and health centres at study locations. Two methods were used for the examination of blood samples (thick smear and concentration). Age grading yielded 42.1% nullipars and 57.9% parous; about 50.0% of the parous females had ovulated once, while 16.25-17.01% ovulated more than twice. The infection rates in An. gambiae s.l. and Cx. quinquefasciatus were 0.10 and 0.02% respectively. The overall infection rate in humans was 4.75%; the infection rates were 5.5 and 0.4% in rural and urban locations respectively. Wuchereria bancrofti, Loa loa and Mansonella persitans were identified; W. bancrofti was dominant. Filariasis prevalence was highest in the ≥60yr age-group and zero in the 0-19yr age-group. Longevity of mosquitoes in both seasons met the threshold for the complete development of the filarial worms to the infective stage. Infection rates in mosquitoes and humans were generally low, with highest prevalence was recorded in rural areas.
Keywords: Wuchereria bancrofti, Culex quinquefasciatus, Anopheles gambiae, BancroftianFilariasis Prevalence, Infection rates, Akwa Ibom State, Humans
Cite this paper: Veronica I. Itina, M. Aline E. Noutcha, Samuel N. Okiwelu, Wuchereria bancrofti Infection Rates in Humans (Vertebrata: Hominidae) and Mosquitoes (Diptera: Culicidae), Akwa Ibom State, Nigeria, Research in Zoology , Vol. 4 No. 1, 2014, pp. 1-7. doi: 10.5923/j.zoology.20140401.01.
Article Outline
1. Introduction
- Lymphatic filariasis is widespread in tropical and subtropical regions. The three causative agents of this disease are: Wuchereria bancrofti, Bruglia malayi and Bruglia timori. The areas of the world endemic for lymphatic filariasis include parts of western, central and southern Africa, parts of northeastern South America, Southeast Asia and Islands of the South Pacific Ocean. There are an estimated 905 million people at risk of contacting lymphatic filariasis and 128 million active infections. Of these, about 115 million are caused by W. bancrofti, the causative agent of Bancroftian filariasis, which is widespread in both the old world and New World tropics[1]. In tropical African surveys, ecological and population studies have highlighted the importance of mosquitoes as vectors of lymphatic filariasis[1, 2, 3]. The contaminated water in pit latrines, septic tanks and drains which constitute good breeding habitats for Culex quinquefasciatus has led to an increase in the prevalence of urban filariasis[4]. The spread of rural filariasis has been enhanced by the development of dams, reservoirs, etc, which are ideal, all-season habitats for the vector, Anopheles gambiae s.l.[5]. An adequate understanding of the dynamic processes underlying infection persistence is an invaluable tool in evaluating progress on the eradication of lymphatic filariasis [6]. There is dearth of information on the epidemiology of Bancroftian filariasis in Akwa Ibom State, Nigeria. A 2-year study was therefore undertaken on filarial infection rates in humans and mosquitoes in urban and rural locations across eco-vegetational zones in the State.
2. Materials and Methods
2.1. Study Area
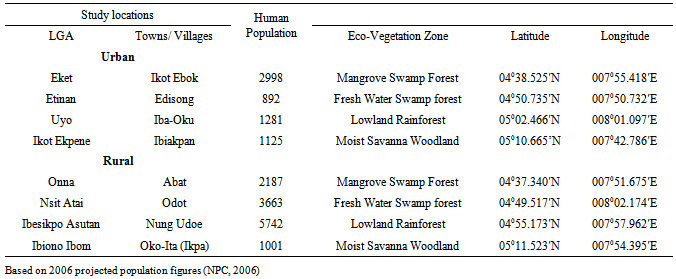
- Akwa Ibom State is in the Southern region of Nigeria, occupying an area of 7,245.935 km2, in four vegetation zones, modified by agriculture (Table1, Figure 1). It lies between 4°32’ - 5°33’N and 7°25’ - 8°25’E, bounded by Abia and Rivers States in the West, South West and North, the Atlantic Ocean in the South, and Cross River State in the East. The four eco-vegetational zones are: 1. The mangrove swamp forests and coastal vegetation are at the estuary of the Qua Iboe River at Eket, Ibeno and Oron, Onna and the creeks of Ikot Abasi, Oruk Anam Local Government Areas.2. The flat lowland rain forests which dominate the state and are prominent at Etinan, Abak and Nsit Atai Local Government Areas.3. The high and undulating moist woodlands of Itu, Ibiono Ibom. Ikono Ikot Ekpene and Ini Local Government Areas.4. The fresh water swamp forests at Uyo and Ibesikpo-Asutan Local Government Areas.There are two seasons: dry (November-March) and rainy (April-October). However the coastal areas tend to have rains year-round.
|
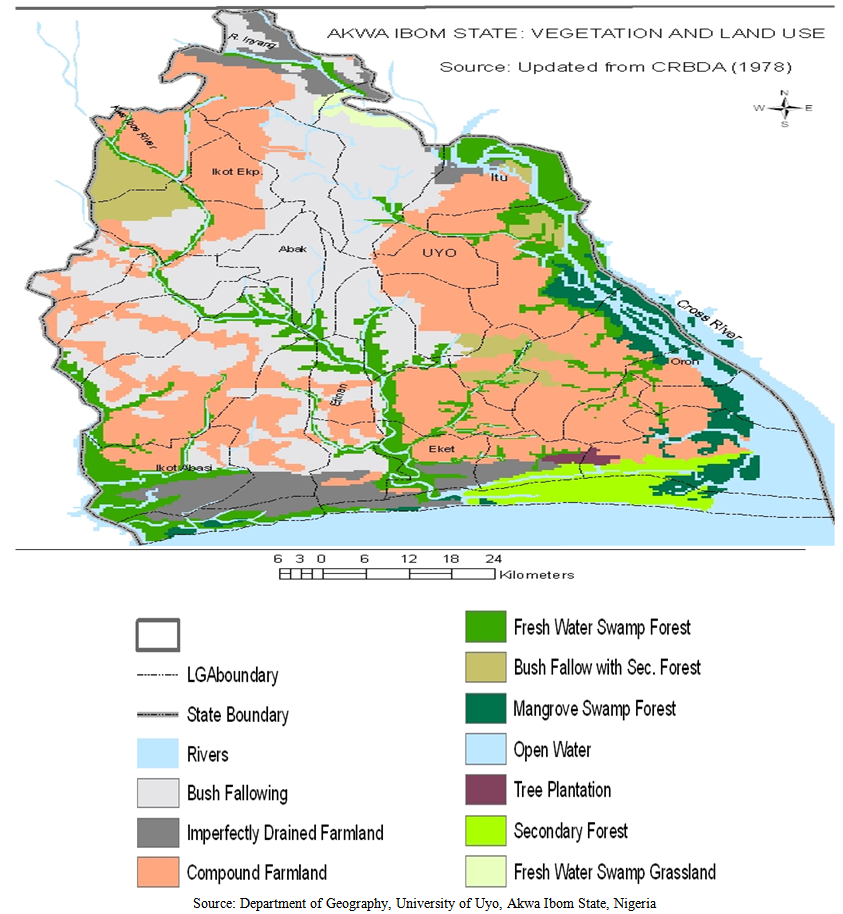 | Figure 1. Akwa Ibom State- Eco-vegetational zones |
2.2. The Study Sites
- The study locations were in eight LGAs (Eket, Onna, Etinan, Nsit-Atai, Uyo, Ibesikpo-Asutan, Okot Ekpene and Ibiono-Ibom) across the four vegetation zones (Mangrove swamps, fresh water swamp forests, lowland rain forest and moist woodland savanna). The urban towns were Ikot Ebok, Edisiong, Iba-Oku and Ibiakpan; the rural villages were Abat, Odot, Nung-Udoe, and Oko-Ita. Populations of the study towns/ villages based on the National Populations Commission (NPC) census data (2006) are shown (Table 1).
2.3. Methods
2.3.1. Mosquito Sampling
- The survey for adult mosquitoes was over a 2-year period, covering 2 rainy and 2 dry seasons. Mobilization was undertaken for 3 months prior to the commencement of collections. Community leaders in the four rural villages (Abat, Odot, Nung-Udoe and Oko-Ita) and the four sections of urban towns (Ikot Ebok: Eket, Edisong: Etinan, Iba-Oku: Uyo, Ibiakpan: Ikot Ekpene) were briefed on the importance of the survey and their cooperation sought and obtained. Houses were randomly selected; majority of these houses were built with bricks and corrugated iron sheets. In the villages, mud houses with corrugated iron sheets were more abundant, although a few houses had thatched roofs. Widths and heights of houses varied with locations. The most practical method for the indoor collection of mosquitoes was by the Spray Catch Method[7]. In each house, a room occupied by residents the previous night was selected. Collections were made, with the help of trained staff from the primary health centres, in the mornings, before 10.00hrs after room occupants had left the house. Food items and relatively small pieces of furniture were removed; two white cotton sheets (2mx2m and 1mx2m) were spread on the floor; flat surfaces of large furniture were completely covered. Windows and doors were firmly closed and all openings blocked. The selected room was sprayed from outside with pyrethrin-based Sheltox BP, through openings below doors and later inside, clockwise on ceilings and walls until the room was filled with the insecticidal mist. On completion, the operator exited and shut the door. After 20min, the door was opened, the collector carefully walked on the sides of sheets, to avoid crushing knocked-down mosquitoes. The sheets were carefully picked up at the corners by two of the three collectors assigned to each study site. Mosquitoes were picked with forceps, placed on soaked cotton wool in containers and covered with filter paper. The containers were later placed in an insulated box with ice packs to ensure that the mosquitoes were properly preserved for dissection. Separate containers, labelled with location and date were used for each location. Mosquitoes were examined for adult features with hand lens and under the dissecting microscope using standard keys[8,9]. The type specimens from the Arbovirus Research Institute, Federal Ministry of Health, Enugu, were used for comparison under the guidance of Mr A.J. Akpan, the Curator of the Insect Museum, Department of Animal and Environmental Biology, University of Port Harcourt.
2.3.2. Dissection of Mosquitoes
- Age-grading is important for comprehensive understanding of the epidemiology of filariasis[10, 11, 12]. The method adopted for the dissection of the mosquitoes was a combination of methods[13, 14, 15]. Ordinarily, all eggs develop synchronously and become mature in two to five days after blood feeding at favourable temperatures. During this time, the more advanced follicle within each ovarioles passes through a series of five easily observed physiological stages (Fig 2), originally described by Christophers[16] and concisely summarized by Clements[17]. The entire cycle of egg production from blood meal through oviposition is the gonotrophic cycle. Parous and nulliparous female mosquitoes were distinguished by the examination of tracheoles of the ovaries. Mosquitoes with a visible follicular relic or sac at the end of the pedicel of at least three ovarioles were recorded as parous while the absence of relics, pigment lumps when the membrane was stretched or the presence of skeins at the end of the ovarian tracheoles indicated nulliparity. This permitted an assessment of the nulliparous: parous ratio, which indicated the probability of survival of mosquitoes; their average longevity was calculated[18]. The data obtained from the physiological age were used to calculate the calendar age, which is a prerequisite for calculating vectorial capacity[19, 10].
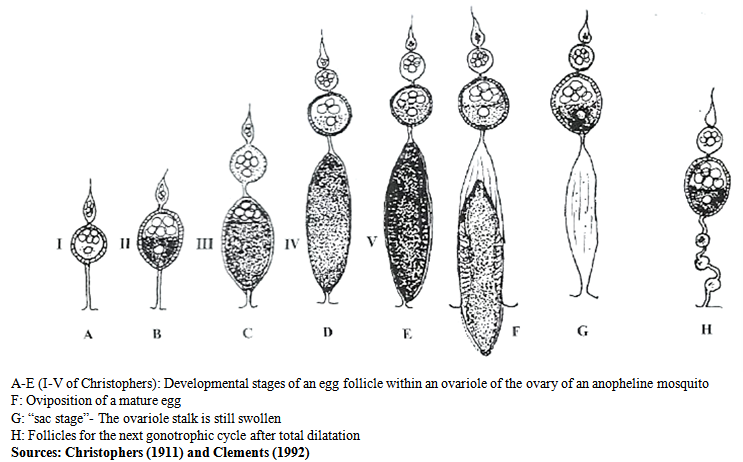 | Figure 2. Egg Follicle development in mosquitoes |
2.3.3. Collection of Blood and Identification of Microfilariae
- Two methods were used for the examination of blood samples (thick smear, concentration). Blood samples were collected with the aid of trained health staff at 10.00 and 15.00 (during the day) and 20.00 and 06.00h (at night) from consenting outpatients in hospitals and health centres at the 8 study locations Abat, Ikot Ebok, Edisiong, Odot, Iba-Oku, Nung-Udoe, Ibiakpan and Oko-Ita. Cotton wool soaked in 70% alcohol was used to clean the tip of the finger; a quick prick was made on the cleaned tip. Blood was collected by filling a 75μl non-heparinized microcapillary tube. This was transferred to a clean slide, labelled with the identification number of patient, location and date. Approximately 50μl blood was dispensed, leaving 25μl in tube. The blood was gently spread over a surface, 25cm x 1cm and stirred for 20seconds to ensure uniform defibrinisation. The slides were dehaemoglobinized by placing them vertically in a staining dish containing methanol for 1 min and stained in 10% Giemsa for 45 minutes. They were dry-washed after staining [22]. Two ml of blood was collected intravenously from the cubital vein of the arm of individuals and transferred into a sterile bottle containing the anti-coagulant, EDTA. The entire content was shaken and poured into a centrifuge tube; 10ml of 2% formalin was added, mixed properly and allowed to stand for 5min to lyse the red blood cells. The tube was centrifuged at 3000rpm and the supernatant poured off. The sediment was added to dilute Giemsa stain. Some of the sediments were transferred with a clean Pasteur pipette into a grease-free slide and covered with a slip and examined under the microscope.The slides were initially examined under 100x 10 magnification and subsequently under higher magnifications for microfilariae. Microfilariae were identified to species level by standard keys[21, 13]. They were counted and numbers recorded on the parasitological forms. Clinical signs were noted; data on sex, age, occupation, weight and date of diagnosis completed the records. Identification of microfilariae was based on the following characters:1. Wuchereria bancrofti: has a sheath; body filled with small, round, discrete nuclei which occur throughout the body; No nuclei in the tail2. Loa loa: has sheath; Nuclei are long and occur more extensively in the body; Nuclei extend right to the tip of the tail3. Mansonella perstans: has sheath; Tail is blunt and ends with a nucleus, portraying a bulb-like appearance.
2.3.4. Data Analysis
- Seasonal variation in nulliparous: parous ration between seasons was subjected to the Chi-square test.
3. Results
3.1. Age-grading and Infection Rates in Anopheles Gambiae s.l and Culex Quinquefasciatus
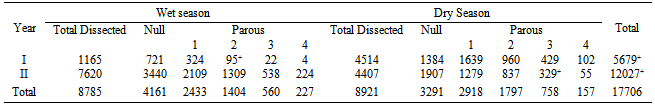
- A total of 17, 706 culicids were dissected for age-grading; 7,452 (42%) were nullipars and 10,254 (57.9%) parous (Table 2). In each season, about 50% of the parous females had ovulated once; 16.25 and 17.01% ovulated more than twice in the dry and rainy seasons respectively (Table 2). Seasonal variation in parous: nulliparous ration was significant (p=0.05; χ2= 199.201). A total of 6873 females were dissected to determine infection rates; 1847 An. gambiae s.l. were dissected, 815 were nullipars while 1032 were parous. One An. gambiae s.l. harboured infective 3rd stage W. bancrofti, an infection rate of 0.10%. A total of 5026 Cx. quinquefasciatus were dissected, 1770 were nullipars and 3,486 were parous. One Cx. quinquefasciatus harboured infective 3rd stage W. bancrofti, an infection rate of 0.02%.
|
3.2. Human Filariasis
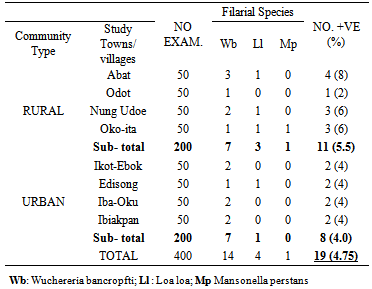
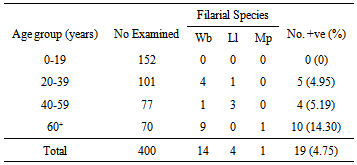
- Four hundred people were examined: 19 persons were positive for microfilariae, for an overall prevalence rate of 4.75% in the study area. In the rural area, 200 people were examined, 11 were positive, a prevalence rate of 5.5%. In the urban locations, 200 people were examined and 8 were positive, a prevalence rate of 0.4%. From the 400 examined, 14 had W. bancrofti, 4 L. loa, and 1 Ma. perstans (Table 3). Filariasis prevalence was highest in the age group ≥ 60yrs; the number positive was 10 (14.30%). No microfilariae were found in the age group 0-19years (Table 4).
|
|
4. Discussion
4.1. Distribution and Age Structure of Mosquito Species
- It is apparent that the distribution of Cx. quinquefasciatus is increasing with urbanization and human activity; many rural pockets that were relatively free of this species are now increasingly colonized[23, 24]. The occurrence of 20.0- 40.0% nullipars in dissected mosquitoes both in the wet and dry seasons confirmed that breeding was continuous throughout the year. It was observed that this percent was lower in the dry season in both Anopheles gambiae s.l. and Culex quinquefasciatus. This was probably related to the reduced number of breeding sites during the dry season. This pattern was observed in the four major eco- vegetational zones. The observed longevity of substantial numbers of female An. gambiae s.l. and Cx. quinquefasciatus was enough for the complete development of Wuchereria bancrofti to the infective 3rd stage larva[25, 26].
4.2. Filarial Infection Rates in Mosquitoes
- Global review on lymphatic filariasis considered Anopheles mosquitoes as principal vectors in West Africa, Papua New Guinea, Vanuatu and Solomon Islands while Cx. quinquefasciatus and other members of the Cx. pipiens complex were the main vectors in China- South Asia, Egypt-East Africa, the Caribbean and Latin America[27]. Cx. quinquefasciatus was described as an urban vector[28] of filariasis, which was maintained in the rural area by An. gambiae[29]. The current results indicate that both species may be important vectors of lymphatic filariasis and with the extensive distribution of Cx. quinquefasciatus, this species may be important in both rural and urban areas.
4.3. Filarial Infection Rates in Humans
- More than 52% prevalence of Bancroftian filariasis was found in the age group ≥60 years and none in the age group 0-19 years. These results are at variance with those of Udonsi in the Niger Delta who observed that the prevalence increased with age, with the highest percent occurrence in the age groups 20-49 and 50-59 years[30, 31]. They are also different from those of Ojiako and Onyeze at Oguta, South Eastern Nigeria where the highest prevalence was in the age group 45-51 years[32]. The current results are similar to those of Braide et al. in Cross River State who found that most positive cases were within the age group 50-70 years[33]. The observed differences may be attributable to type of species involved[31]; diversity in environmental conditions[34, 35] and differences in the occupation of those examined[30].
5. Conclusions
- Longevity of mosquitoes in both seasons was within the range for the complete development of the filarial worms to the infective stage. Infection rates in mosquitoes and humans were generally low, although higher prevalence was encountered in rural areas.
ACKNOWLEDGEMENTS
- Dr V. I. Itina acknowledges with gratitude the support of staff in the Ministry of Health, Akwa Ibom State, and advice from Prof O.C. Umeozor. The assistance of Mr A. J. Akpan (late) in field collections and mosquito identification is highly appreciated. The cooperation of community leaders, guides, field escorts and health workers at the various health centres at study locations is gratefully acknowledged.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML