-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
p-ISSN: 2325-002X e-ISSN: 2325-0038
2013; 3(2): 62-65
doi:10.5923/j.zoology.20130302.03
Spatial and Temporal Distribution of Tabanids (Diptera: Tabanidae) in Akwa Ibom State, Nigeria
Veronica I. Itina, Aline M. E. Noutcha, Samuel N. Okiwelu
Department of Animal and Environmental Biology, University of Port Harcourt, Port Harcourt, Nigeria
Correspondence to: Samuel N. Okiwelu, Department of Animal and Environmental Biology, University of Port Harcourt, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Rapid Assessment Surveys confirmed the endemicity and widespread occurrence of the tabanid-transmitted human disease, Loiasis in Akwa-Ibom State, Nigeria, but data on the species composition, seasonal abundance and distribution of tabanids in the State was non-existent. A study was therefore undertaken over a 12-month period, covering wet and dry seasons, at rural and urban sites to determine the occurrence, seasonal abundance of known and potential tabanid species across the eco-vegetational zones (Mangrove Swamp Forest, Freshwater Swamp Forest, Lowland Rainforest, Moist Savanna Woodland) in the State. The Malaise trap was the sampling tool. Distribution of tabanids was across all eco-vegetational zones; however, most were collected from the mangrove swamp forest. The mangrove and fresh water swamp forests yielded more than 90% of all tabanids. The total number of tabanids was 2,790; eleven species in four genera (Tabanus, Chrysops, Haematopota, Hippocentrum). The genus Chrysops was dominant, constituting 65.4% of all tabanids; Chrysops silacea, a known vector of Loiasis was the most abundant and widespread species, 32.4% of all tabanids. More than 80.0% were collected in the wet season. Most species occurred in both seasons, with the exception of Tabanus atratus and Chrysops pikei recorded in the wet season only. More than 60.0% of all tabanids were from rural locations. The endemicity and widespread occurrence of loiasis in the State ie: probably maintained by the widely distributed C. silacea and other factors. The rainy season can be considered as the period of potentially higher risk of transmission, particularly in the rural mangrove and fresh water swamp forests.
Keywords: Tabanids, Loiasis, Distribution, Eco-vegetational zones, Akwa-Ibom State, Nigeria
Cite this paper: Veronica I. Itina, Aline M. E. Noutcha, Samuel N. Okiwelu, Spatial and Temporal Distribution of Tabanids (Diptera: Tabanidae) in Akwa Ibom State, Nigeria, Research in Zoology , Vol. 3 No. 2, 2013, pp. 62-65. doi: 10.5923/j.zoology.20130302.03.
Article Outline
1. Introduction
- Loiasis is a tabanid-transmitted helminth disease of Central and Western Africa[1, 2]. The diurnally periodic microfilariae are usually absent from peripheral blood of people at night, but appear during the day, particularly in the morning. The microfilariae are therefore readily picked up by the primary tabanid vectors which bite during the day[3]. Loiasis has been established as endemic and widespread in Akwa-Ibom State, Nigeria[4-6]. There have been anecdotal records of tabanids in the State. A study was therefore undertaken over a 1-year period to determine variations in species composition, relative abundance and distribution of tabanids in rural and urban locations across the eco-vegetational zones in the State.
2. Materials and Methods
2.1. Study Area
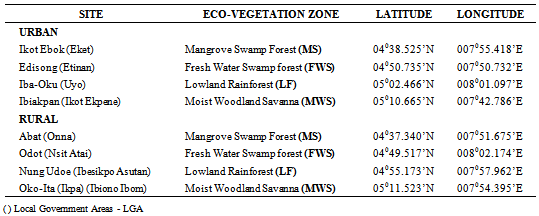
- Akwa Ibom State is located in the south eastern region of Nigeria, 4.320 -5.330N and 7.250 - 8.250E. There are four vegetation zones: mangrove swamp forest, fresh water swamp forest, Lowland rainforest and moist savanna woodland. The mean annual rainfall is 2000-2500mm and the temperature range, 27 - 32℃. There are two seasons: rainy (April - October) and dry (Nov - March).
2.2. Study Sites
- Two Local Government Areas (LGAs), each from an urban and a rural location were randomly selected in each of the four eco-vegetational zones; the LGAs were: Eket, Onna, Etinan, Nsit Atai, Uyo, Ibesikpo Asutan, Ikot Ekpene and Ibiono-Ibom. The geographical coordinates of the study towns and villages appear in Table 1.
2.3. Vector Collection, Handling and Identification
- The Malaise trap, effective for the collection of tabanids[7] was used. Tabanids were collected fortnightly, 07.00-19.00hrs; data were pooled to obtain monthly relative abundance.
|
2.4. Data Analyses
- SPSS (Graph path) was used for data analyses. The Students’t-test and the Analysis of Variance (ANOVA) were used to analyze seasonal, rural-urban and vegetational data. All statistical tests were performed at 5% (0.05) level of significance.
3. Results
3.1. Tabanid Distribution across Eco-vegetational Zones
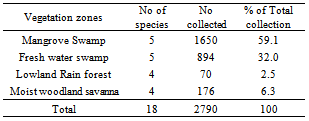
- The total number of tabanids collected in the 4 vegetation zones was 2790. The mangrove swamp produced the highest number 1650 (59.1%); the least was from Lowland rainforest, 70 (2.5%) (Table 2). Differences in numbers across eco-vegetational zones were not significant (F = 0.61 < 4.35, df 3, 4; P = 0.05).
3.2. Distribution of Tabanids in Rural and Urban Locations
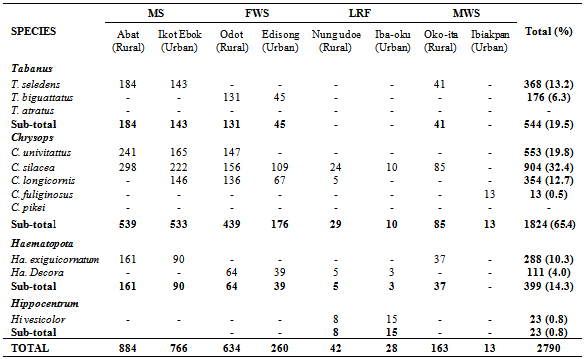
- More Tabanids were collected from the rural areas, constituting 63.0% of all collections. This pattern was observed in all species, except C. fuliginosus which was confined to urban locations (Table 3). However, the difference in rural-urban numbers was not significant (t = 1.98 < 2.131; df = 15, P > 0.05).
3.3. Seasonal Abundance of Tabanids
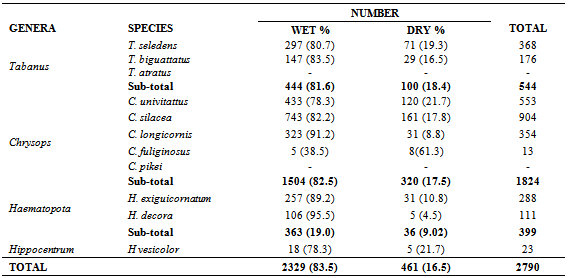
- In the wet season, April, - September, 2329 (83.5%) were collected, while 461 (16.5%) were recorded in the dry season (October-March). This seasonal activity pattern was observed in all species, except Chrysops fuliginosus (Table 4). Some species were absent in some months of the dry season: C. univattatus (February), C. silacea(December-February). C. longicornis (February -March); C. fuliginosus (January-June), H. exiguicornatum (January-February); H. decora (November-February) and Hippocentrum vesicolor (December-February). However, the overall seasonal differences were not significant (t = 1.64 > 2.447; df = 6, P > 0.05).
4. Discussion
- The preponderance of tabanids in the mangrove and freshwater swamps is not surprising because the oviposition sites overhang or are adjacent to the larval habitats, which are often muddy, aquatic or semi-aquatic habitats[3]. They are also known to breed in brackish swamps[11]. The more complex rural locations in these eco-vegetational zones were also preferred[2]. Species richness of tabanids was found to negatively correlate with large open habitats and positively with patch-shape complexity[12]. Rains provide ideal breeding grounds and the higher numbers in the wet season have also been observed in other studies[13-17]. The population peak of most species including those with higher vector potential suggests that the rainy season can be considered as the period of potentially higher risk of transmission[12].
|
|
|
5. Conclusions
- The favourability of muddy habitats for tabanids oviposition is reflected in their preponderance in swamp forests. The patterns of spatial distribution of Chrysops silacea suggest that the disease may be more prevalent in rural communities in the wet season. The endemicity and widespread occurrence of loiasis in Akwa Ibom State is probably maintained by the widely distributed C. silacea and other vectors, enhanced by their high dispersal ability.
ACKNOWLEDGEMENTS
- Dr V. I. Itina acknowledges with gratitude the support of staff of the Ministry of Health, Akwa Ibom State, and advice from Prof O.C. Umeozor. The assistance of Mr A. J. Akpan in field collections and mosquito identification is highly appreciated. The cooperation of community leaders, guides, field escorts and health workers at the various health centers at study locations is gratefully acknowledged.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML