-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
p-ISSN: 2325-002X e-ISSN: 2325-0038
2013; 3(2): 56-61
doi:10.5923/j.zoology.20130302.02
Comparative Biometry of the Iranian Cichlid, Iranocichla hormuzensis, in Different Seasons and Sexes
Ehsan Daneshvar, Yazdan Keivany, Elmira Paknehad
Department of Natural Resources (Fisheries Division), Isfahan University of Technology, Isfahan, 84156-83111, Iran
Correspondence to: Yazdan Keivany, Department of Natural Resources (Fisheries Division), Isfahan University of Technology, Isfahan, 84156-83111, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
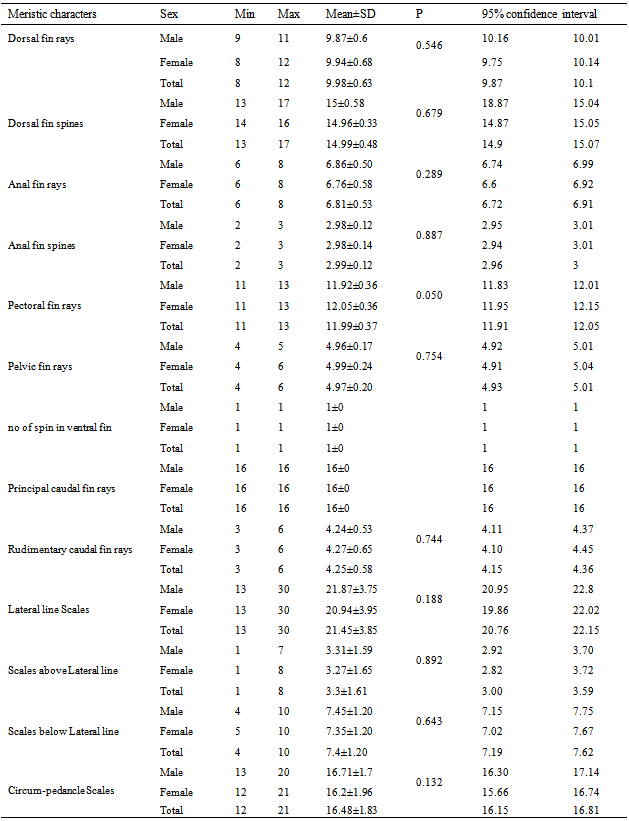
Biometry of Iranocichlahormuzensis was compared between males and females and two seasons. A total of 120 specimens were collected from Mehran River and 25 morphometric and 13 meristic characters were measured. All mean values of the morphometric characters, except eye diameter and pectoral fin base length, were significantly higher in males (p<0.05). To analyze the effects of seasonal change on biometric characters, these characters were compared in wet and cooler (October-March) and dry and warmer (April-September) seasons for males and females. All morphometric characters increased significantly (p<0.05) from wet to dry season, although females were more affected by season than males. The correlation between morphometric measurements and total length, except for the pectoral fin base length, were high. There were no significant differences in meristic characters between males and females of I. hormuzensis. Comparison between meristic characters in different seasons showed that rudimentary caudal fin rays and scales above the lateral line in dry season were significantly higher than in wet season for both sexes(p<0.05). Sex and season did not significantly affect the meristics of the Iranian cichlid, however, morphometricswere highly affected by these factors and should be considered in comparative biology studies.
Keywords: Cichlidae, Hormuz Basin, Morphometrics, Meristics
Cite this paper: Ehsan Daneshvar, Yazdan Keivany, Elmira Paknehad, Comparative Biometry of the Iranian Cichlid, Iranocichla hormuzensis, in Different Seasons and Sexes, Research in Zoology , Vol. 3 No. 2, 2013, pp. 56-61. doi: 10.5923/j.zoology.20130302.02.
Article Outline
1. Introduction
- Iranocichlahormuzensis, the only native cichlid in Iran[1,2]) (Figure 1), is a small fish, usually eaten by local people whenavailable in large numbers during spring[3]. Iranian cichlid has a terminal mouth and in aquaria, feed from surface, bottom and water columns. Iranocichlahormuzensis is easily recognized by the single nostril opening on each side of the head. Ctenoid Scales are regularly arranged on the flanks, but may be entirely absent from some parts of the body. The lateral line is divided into two parts, an anterior and higher portion and a lower, posterior portion.Morphometric characters describe aspects of body shape and meristicare the number ofcountable structures that are fixed in embryos or larvae[4]. Morphometrics and meristicshave been used to identify fishspecies andtheirhabitat specificity, as well as, ecological criteria in streams, lakes or seas[5,6]. The morphometry of fishes is used for characterizing strains of one species[7], for stock identification[8] and assessing the evolutionary adaptation of a species to its environment[9]. Morphometric characters have been suggested as potential means for identifying thresholds in the life history of fishes[10,11]. Specimens from different areas have different morphology and knowledge of biometric variations is useful in descriptions of the species[12].This paper provides the first information on morphometric and meristic characters of I. hormuzensis for male and female in Mehran River, and compares these characters between wet and cooler (October to March, 20.8±3.2°C) and dry and warmer (April to September, 32.2±2.3°C) seasons.
 | Figure 1. A photo of Iranocichlahormuzensisfrom Mehran River |
2. Materials and Methods
- A total of 120 specimens (66 males and 54 females) of Iranocichlahormuzensis from Mehran River at 26°52´53˝, 55°16´21˝E 31 m altitude, in HormuzganProvince (Figure 2), were collected by a seine net (8 m×1 m, 5 mm mesh size) from September 2008 through August 2009. The specimens were transferred to the Ichthyology Laboratory of Fisheries Division, Isfahan University of Technology, where all morphometric and meristic characteristics were carried out. Specimens were measured with a digital calliper to the nearest 0.01 mm and weighed with an electric scale to the nearest 0.01g.
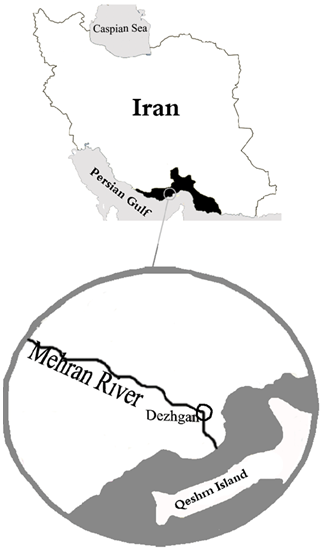 | Figure 2. Collection site of Iranocichlahormuzensisfrom Mehran River |
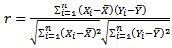 | (1) |
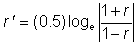 | (2) |
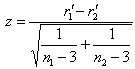 | (3) |
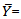 mean total length of population, Xi= value of each other morphometric character,
mean total length of population, Xi= value of each other morphometric character, 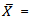 mean of each morphometric character of population, n1 number of males and n2 number of females. A simple linear regression model was used to plot the dependent variables against the total length.
mean of each morphometric character of population, n1 number of males and n2 number of females. A simple linear regression model was used to plot the dependent variables against the total length.3. Results
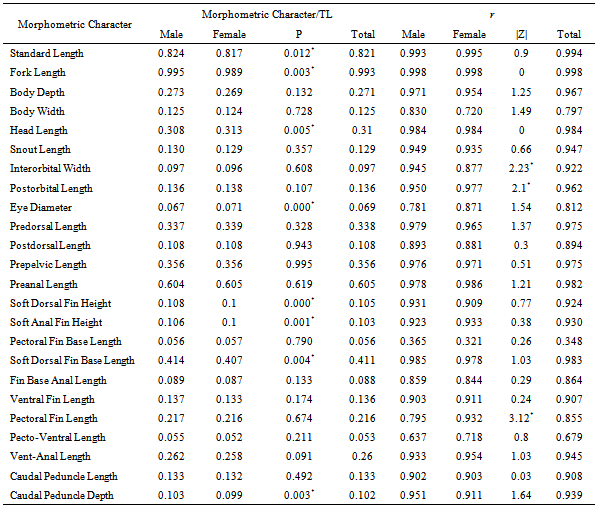
- A total of 120 specimens (66 males and 54 females) of I. hormuzensis ranging between 35.88-91.79 mm in total length (TL) and 0.78-16.9 g in weight, were used for morphometric and meristic analyses. Males ranged from 37.98 to 91.79 mm (63.53±10.53) in total length and from 0.97 to 16.09 g (5.48±2.81) in weight. Females ranged from 35.88 to 84.43 mm (61.40±6.47) in total length and from 0.78 to 11.12 g (3.7±1.85) in weight. Table 1 presents relative morphometric measurements for males, females and combined sexes of I. hormuzensis. Morphometric characters were compared between males and females. Except eye diameter and pectoral fin base length which were not different between the sexes, males mean values of other characters were significantly higher than those of females (t-test, P<0.05). Head length values were19.58±3.42 for males and 18.14±10.96 mm for females, indicating a significantly largerhead in the males (t-test, P<0.05).Morphometric characters were compared between wet and cooler (October to March, 20.8±3.2 °C) and dry and warmer (April to September, 32.2±2.3 °C) seasons for males and females to observe the effects of seasonal changes on these characters. All characters that were different between the two seasons, showed significantly higher values in dry season for both males and females. Nine characters for males (standard length, body depth, body width, head length, postdorsal length, soft anal fin height, fin base anal length, ventral fin length and caudal peduncle length) and 18 characters for females (total, standard, and fork lengths, body depth and width, headand snout lengths, interorbital width, postorbital, predorsal, postdorsal, prepelvic, preanal, soft dorsal fin base, ventral Fin, pecto-ventral, vent-anal, and caudal peduncle lengths) were significantly different between dry and wet seasons (t-test, P<0.05).The relative values of standard, fork, and head length, eye diameter, soft dorsal fin height, soft anal fin height, soft dorsal fin base length and caudal peduncle depth, against the total length, were significantly different between males and females.
|
|
4. Discussion
- These data are the first report on the morphometric and meristic differences of Iranocichlahormuzensis in males and females and in different seasons (wet and dry).These results show that except for the eye diameter and pectoral fin base length, the other morphological characters are different between the two sexes. It is noted that larger males are common in riverine and lagoonal populations of cichlids[16] and it was attributed to the fact that sperm production needs less energy than egg production[17]. Such a sex differences are reported in other fishes[18-26]. Differences in sizes of males and females could be related to spatial segregation of the sexes[27]. In cichlid fishes, it is a selective advantage during the reproductive season if males can defend a nest or brood against potential predators[27].All morphometric characters with significant differences in different seasons, increased from wet to dry season for both males and females. Differences in morphometric characters between the two seasons could be related to sexual activity; in February to June, males consumemore energy to make nests and protect them and females consumed more energy for egg production and maintain offspring. The 19 significantly different morphometrics for females and 10 for males in wet and dry seasons, indicated that females are more affected by seasonal changes than males.Comparisons between relative values of morphometric measurements showed that the ratio of standard length, fork length, soft dorsal fin height, soft anal fin height, soft dorsal fin base length and caudal peduncle depth to total length in males are significantly higher than in females. On the other hand, in females,head length andeye diameter/total length showed higher values than those in males. Although head lengths of males were significantly larger than females (t-test P<0.05), but eye diameter values did not significantly differ between males and females(t-test P>0.05). Ratio of head length and eye diameter to total length of females were larger than males. As a mouth breeder fish, after mating, females incubatetheiroffsprings in their mouth. The reason for having a larger head in females could be related to females need for more space to hold eggs and larva. Also, larger eyes in females may be related to the greater role of females to protect the progenies against predators. A longer body, higher soft dorsal and soft anal fins and deeper caudal peduncle in males could be related to having more and faster swimming in males. In the present study we found no significant sex differences in meristic characters of I. hormuzensis. Different numbers of rudimentary caudal fin rays in the two seasons may be due to a faster growth in dry season.
5. Conclusions
- In conclusion, the sex and season do not significantly affect the meristics of the Iranian cichlid, however, morphometrics are highly affected by these factors both by sex and season and should be considered in comparative biology studies.
ACKNOWLEDGEMENTS
- The authors would like to thank Dr. N.M. Soofiani, dean of Faculty of Natural Resources, for providing laboratory facilities and space, Drs. E. Ebrahimi, S. Dorafshan and BSc. students N. Gilannejad, M. Yazdani, B. Tavakoli, V. Kiani, G. Sharifi and S. Kouhie, for their help in laboratory work and fish collection and the fishery laboratory assistances Mr. S. Asadollah, Mr. E. Motaghi and Ms. N. Rajaei. This work was financial supported by the Student Affairs of the Isfahan University of Technology.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML