-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
p-ISSN: 2325-002X e-ISSN: 2325-0038
2013; 3(2): 45-55
doi:10.5923/j.zoology.20130302.01
Spermatogenesis, Spermiogenesis and Sperm Transfer in Two Freshwater Leeches from Assiut, Egypt
Hanaa A. Gouda
Zoology Department, Faculty of Science, Assiut University, Assiut, Egypt
Correspondence to: Hanaa A. Gouda, Zoology Department, Faculty of Science, Assiut University, Assiut, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Spermatogenesis and spermiogenesis, in two freshwater leeches; Helobdella stagnalis and Salifa delicata, were studied by light and electron microscopy. Within the testisacs large polygonal spermatogonia, measuring 6.25 x 5.25 um, divided mitotically to form isogenic groups and, finally, primary spermatocytes. The cells were all clustered in groups, evolved gradually and synchronically, remaining connected by cytoplasmic bridges to a central cytoplasmic mass called the cytophore. The last meiotic division gave rise to spermatids. By means of a complex spermiogenic process of cellular differentiation, each cell became a filiform and corkscrew spermatozoon. The morphogenetic sequence of the various regions of the spermatozoon could be appeared externally by SEM as four different regions. These regions were mentioned, as the same order of their formation, as following: 1) Smooth straight flagellum elongation; 2) Smooth straight mitochondrial elongation region; 3) Right-handed, closely coiled, double helix nuclear spiralization and elongation; and finally, 4) spiralization and elongation, first of the posterior acrosome and after of the less coiled anterior acrosome. Sperm transfer in Salifa delicata occurred through copulation and insertion into a vaginal sac of the partner, while in Helobdella stagnalis, by way of packaged spermatophores implanted indiscriminately through the cuticle of a recipient mate.
Keywords: Helobdella, Salifa, Fine Structure, Spermatogenesis, Spermiogenesis, Egypt
Cite this paper: Hanaa A. Gouda, Spermatogenesis, Spermiogenesis and Sperm Transfer in Two Freshwater Leeches from Assiut, Egypt, Research in Zoology , Vol. 3 No. 2, 2013, pp. 45-55. doi: 10.5923/j.zoology.20130302.01.
Article Outline
1. Introduction
- Leeches are hermaphrodite euclitellates. The ova are fertilized in the female genital system by spermatozoa transferred during copulation, as in some Arhychobdellida, or injected through the body wall as in Rhynchobdellida[1]. Spermatogenesis in leeches begins with the release of epithelial cells from the walls of specialized coelomic sacs; testisacs. The testisacs are found on either side intersegmentally. The released spermatogonia float freely in the lumen of the coelomic sacs wherein they undergo mitotic divisions followed by meiosis without cytokinesis and the haploid nuclei eventually are found arranged around the periphery of an anucleate cytophore to which the gametes remain connected. In spermatogenesis all cells develop into spermatozoa, as opposed to oogenesis where one of the cells matures at the expense of the remaining trophocytes. The spermatocytes develop into mature spermatozoa, detach from the cytophore and remain in bundles held together by glandular secretions[2]. When spermiogenesis is completed the mature spermatozoa are released from the cytophores and pass to the deferent ducts, hence the atria and the male genital pores[3].Information about leeches' spermatozoa is poor and is based on relatively few papers[4-6]. On the other hand, the spermatozoa of leeches are by far the most complex euclitellate spermatozoa and are an interesting field of study to test the mutual interactions of systematic position and physiology of fertilization in determining sperm morphology. This study concerns the spermatogenesis, spermiogenesis and sperm transfer in two different species; Helobdella stagnalis (Rhynchobdellida) and Salifa delicata(Arhynchobdellida).
2. Materials and Methods
- Mature leeches; Helobdella stagnalis and Salifa delicata were collected from Al-Sont canal
 . Maturity and the anatomy of male genital system in both studied leeches could be obtained as shown in[7,8]. Light and electron microscopySix mature leeches were relaxed from each studied species; two were dissected to demonstrate the testisacs and male ducts, fixed in 70 % ethanol and sent to the scanning electron microscope (SEM) unit to examine the different developmental stages and vasa differentia. The SEM specimens were stuck onto a holder in suitable directions using a suitable glue then examined and photomicrographed with Jeol SM (JSM-5400 LV scanning electron microscope), at the EM Unit. The remaining four specimens were cut longitudinally into two halves at the second third of the body. These tissues of both studied leeches were fixed in formol saline (1 g calcium chloride + 100 ml neutral formalin) for 48 hrs. Two specimens were used for paraffin sections. Paraffin tissues were processed routinely for light microscopy. Tissues were sectioned at 6 um and stained in haematoxylin – eosin, Masson's trichrome and periodic acid-Schiff (PAS) - haematoxylin. For transmission electron microscopy, the last two specimens were placed in 2.5% glutaraldehyde in 0.1 M phosphate buffer at pH 7.2 for 3 hrs. at room temperature. The specimens were post fixed in 1% buffered osmium tetroxide at pH 7.2 for 3 hours at 4℃. They were then dehydrated and embedded in Epon. Semi-thin sections of 1.0 um were cut and stained with toluidine blue for examination under light microscope. Ultra-thin sections from the selected areas of the trimmed blocks were made and collected in copper grids. The ultra-thin sections were contrasted in uranyl acetate for 10 minutes, lead citrate for 5 minutes and examined under transmission electron microscope JEM, 100 Cx11 TEM, at Assiut University.
. Maturity and the anatomy of male genital system in both studied leeches could be obtained as shown in[7,8]. Light and electron microscopySix mature leeches were relaxed from each studied species; two were dissected to demonstrate the testisacs and male ducts, fixed in 70 % ethanol and sent to the scanning electron microscope (SEM) unit to examine the different developmental stages and vasa differentia. The SEM specimens were stuck onto a holder in suitable directions using a suitable glue then examined and photomicrographed with Jeol SM (JSM-5400 LV scanning electron microscope), at the EM Unit. The remaining four specimens were cut longitudinally into two halves at the second third of the body. These tissues of both studied leeches were fixed in formol saline (1 g calcium chloride + 100 ml neutral formalin) for 48 hrs. Two specimens were used for paraffin sections. Paraffin tissues were processed routinely for light microscopy. Tissues were sectioned at 6 um and stained in haematoxylin – eosin, Masson's trichrome and periodic acid-Schiff (PAS) - haematoxylin. For transmission electron microscopy, the last two specimens were placed in 2.5% glutaraldehyde in 0.1 M phosphate buffer at pH 7.2 for 3 hrs. at room temperature. The specimens were post fixed in 1% buffered osmium tetroxide at pH 7.2 for 3 hours at 4℃. They were then dehydrated and embedded in Epon. Semi-thin sections of 1.0 um were cut and stained with toluidine blue for examination under light microscope. Ultra-thin sections from the selected areas of the trimmed blocks were made and collected in copper grids. The ultra-thin sections were contrasted in uranyl acetate for 10 minutes, lead citrate for 5 minutes and examined under transmission electron microscope JEM, 100 Cx11 TEM, at Assiut University. 3. Results and Discussion
3.1. Spermatogenesis
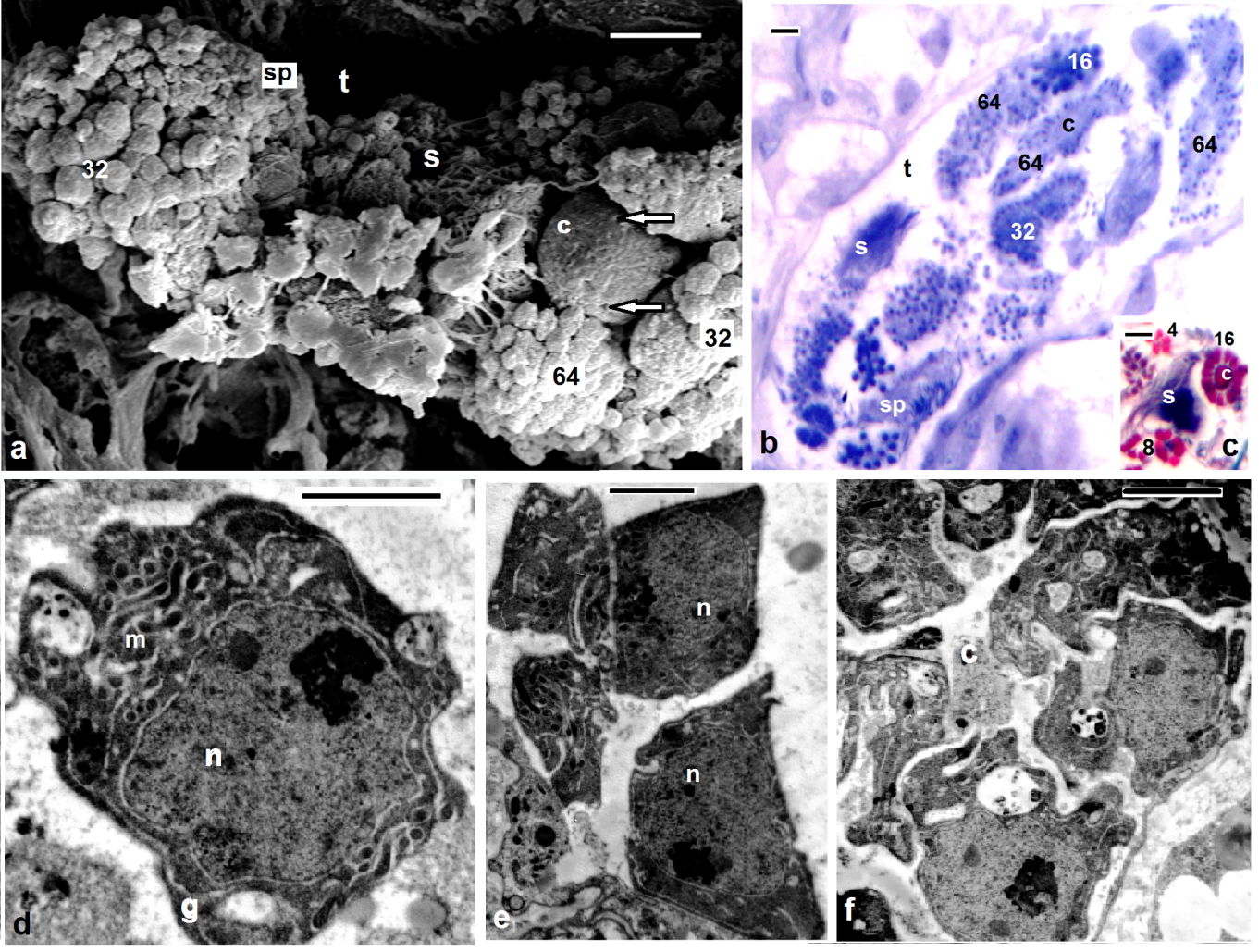
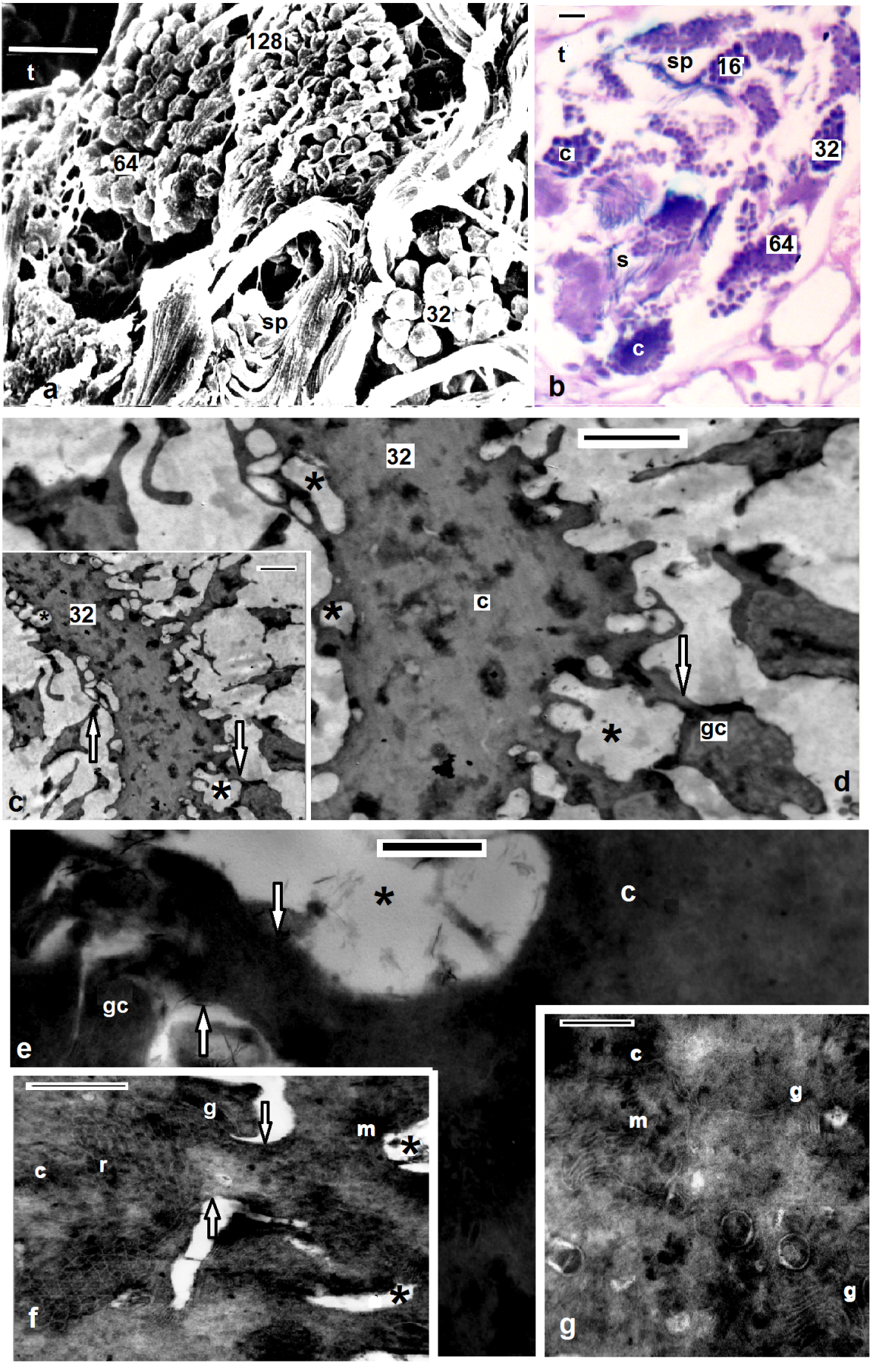
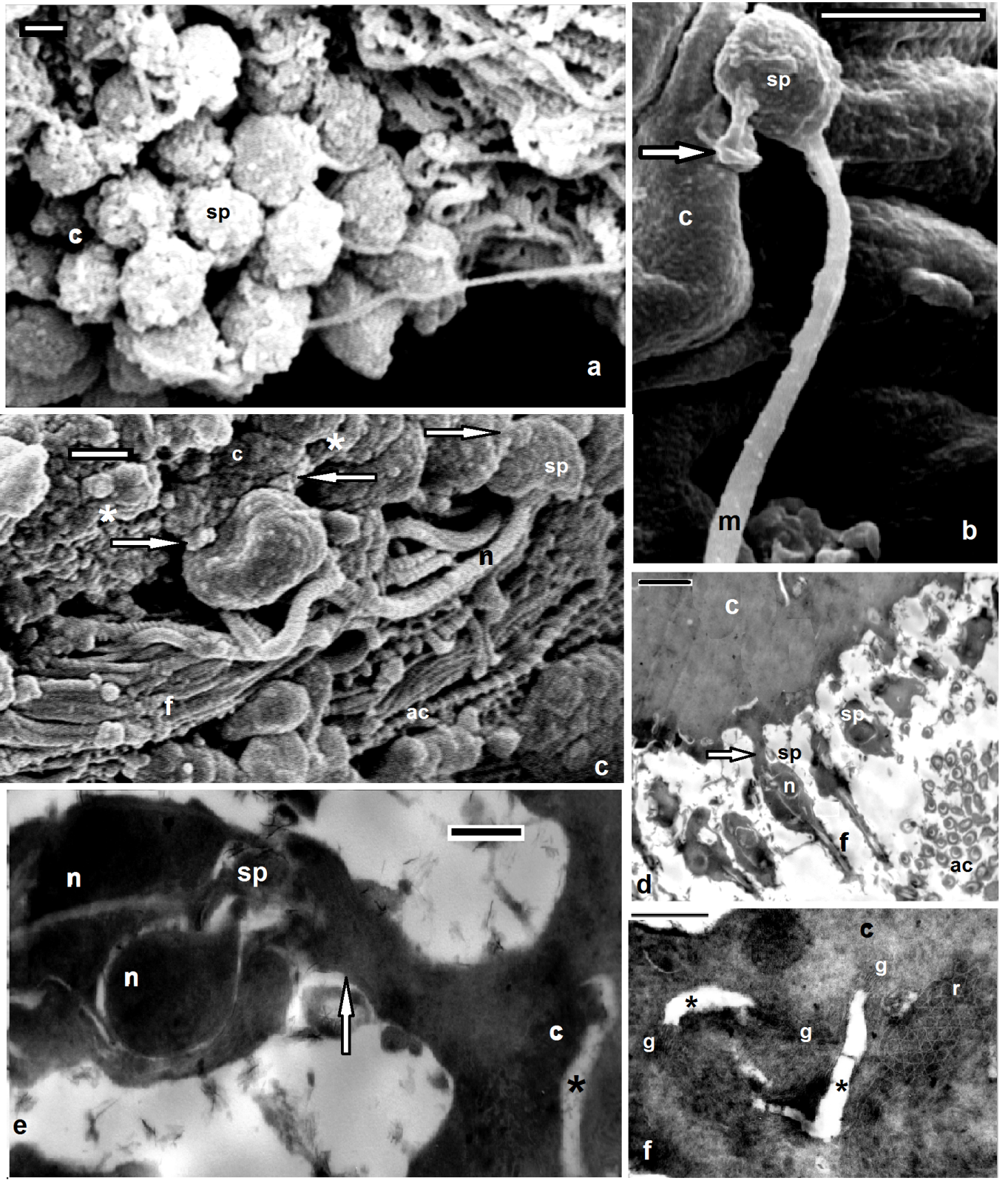
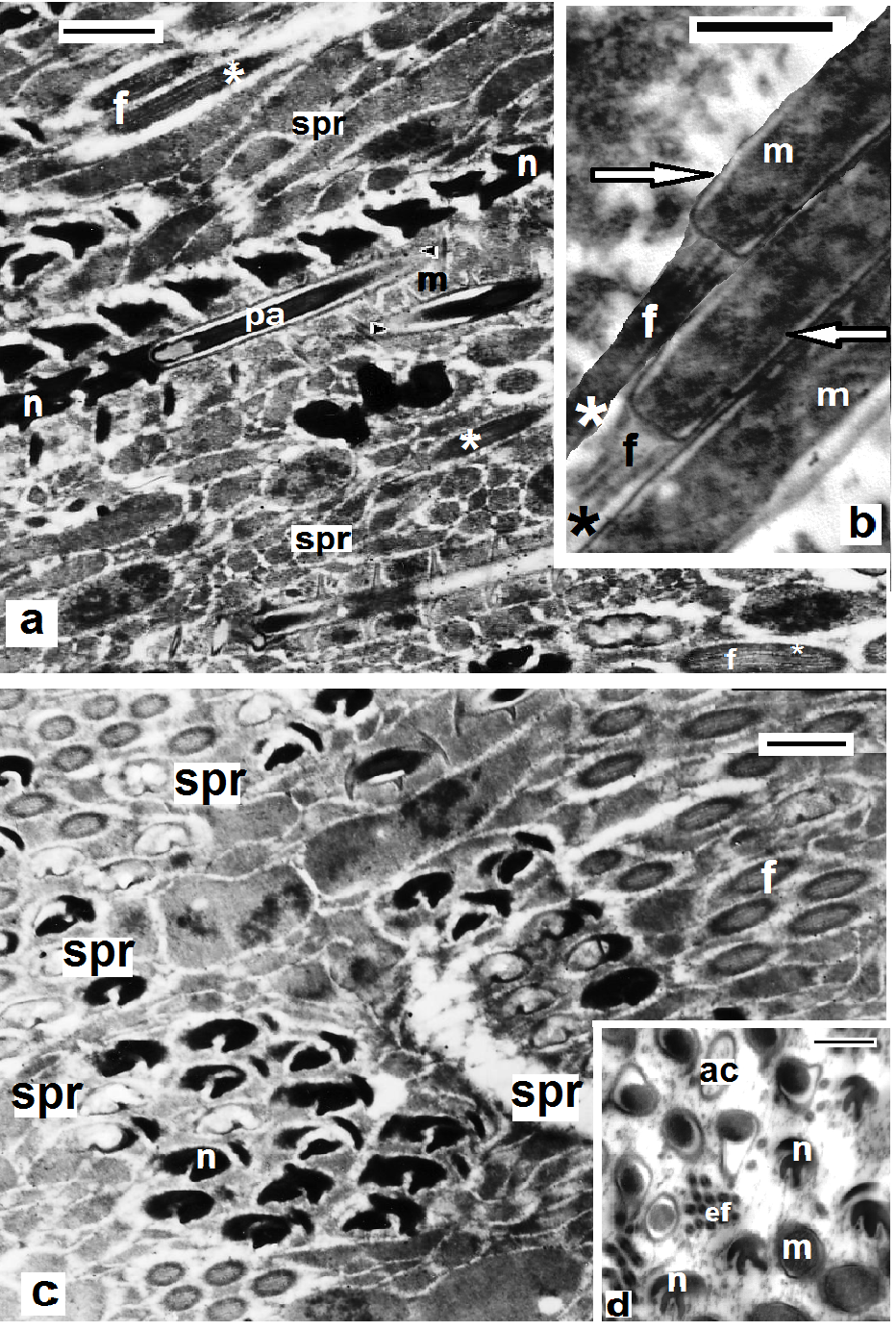
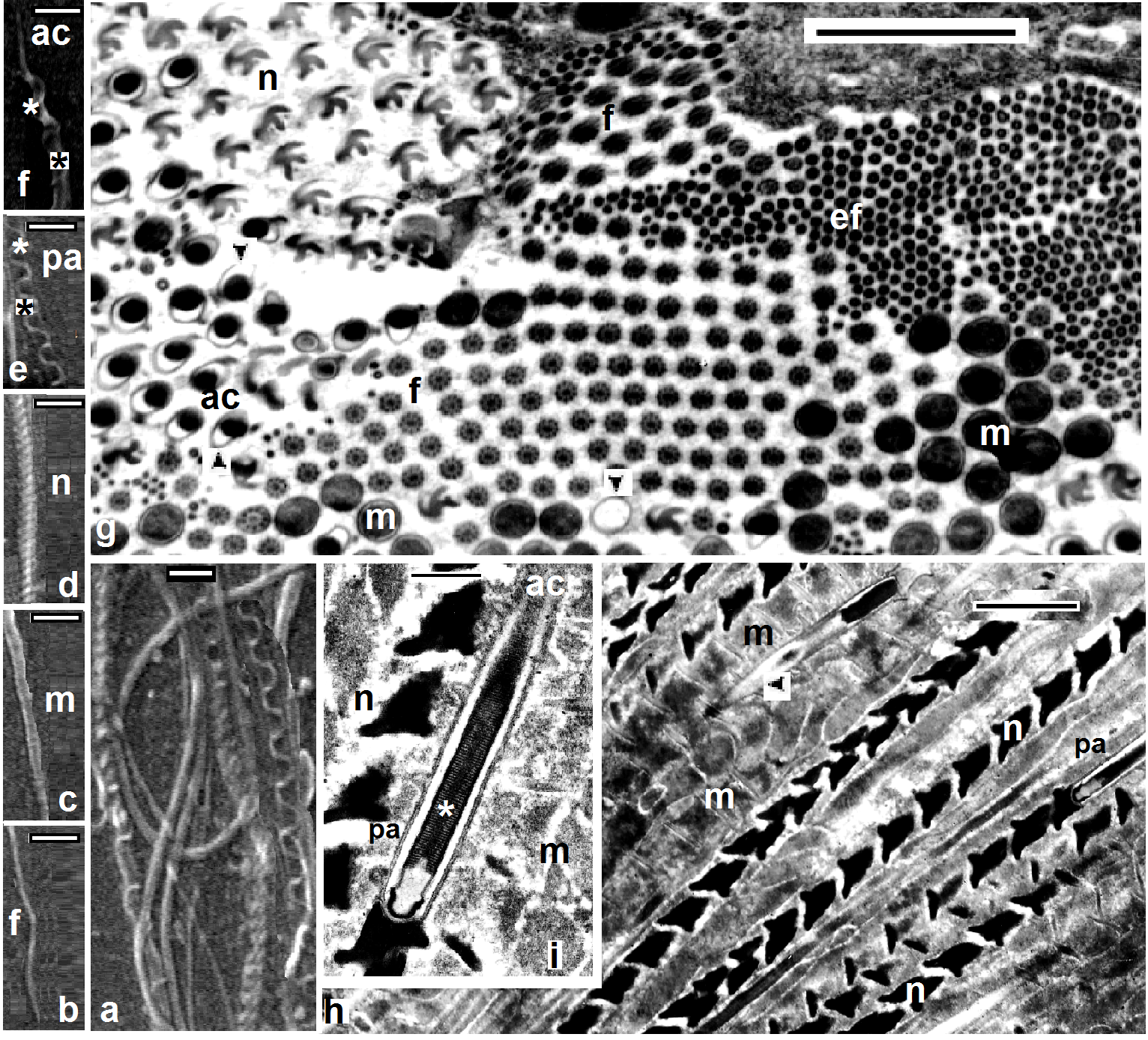
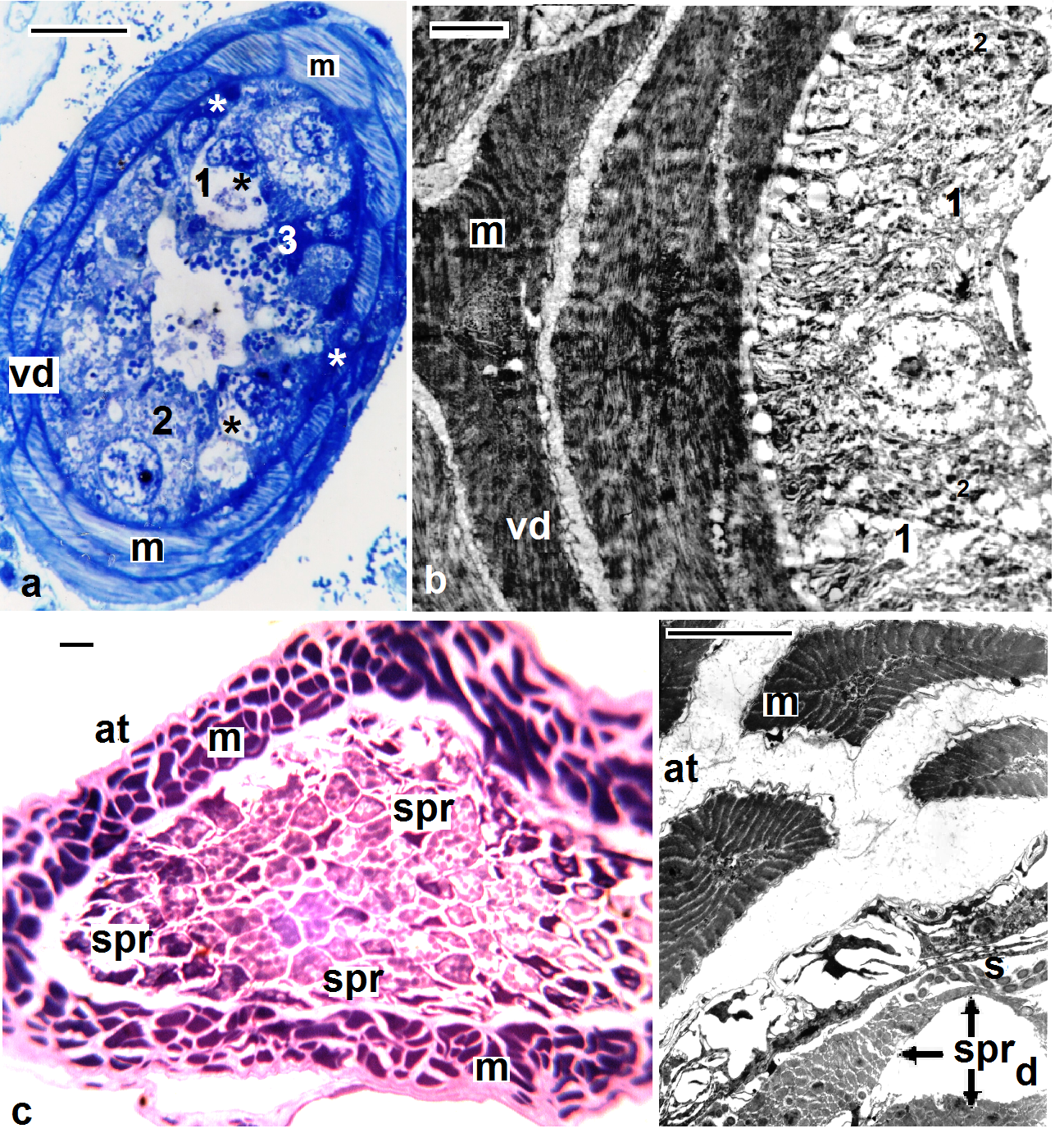
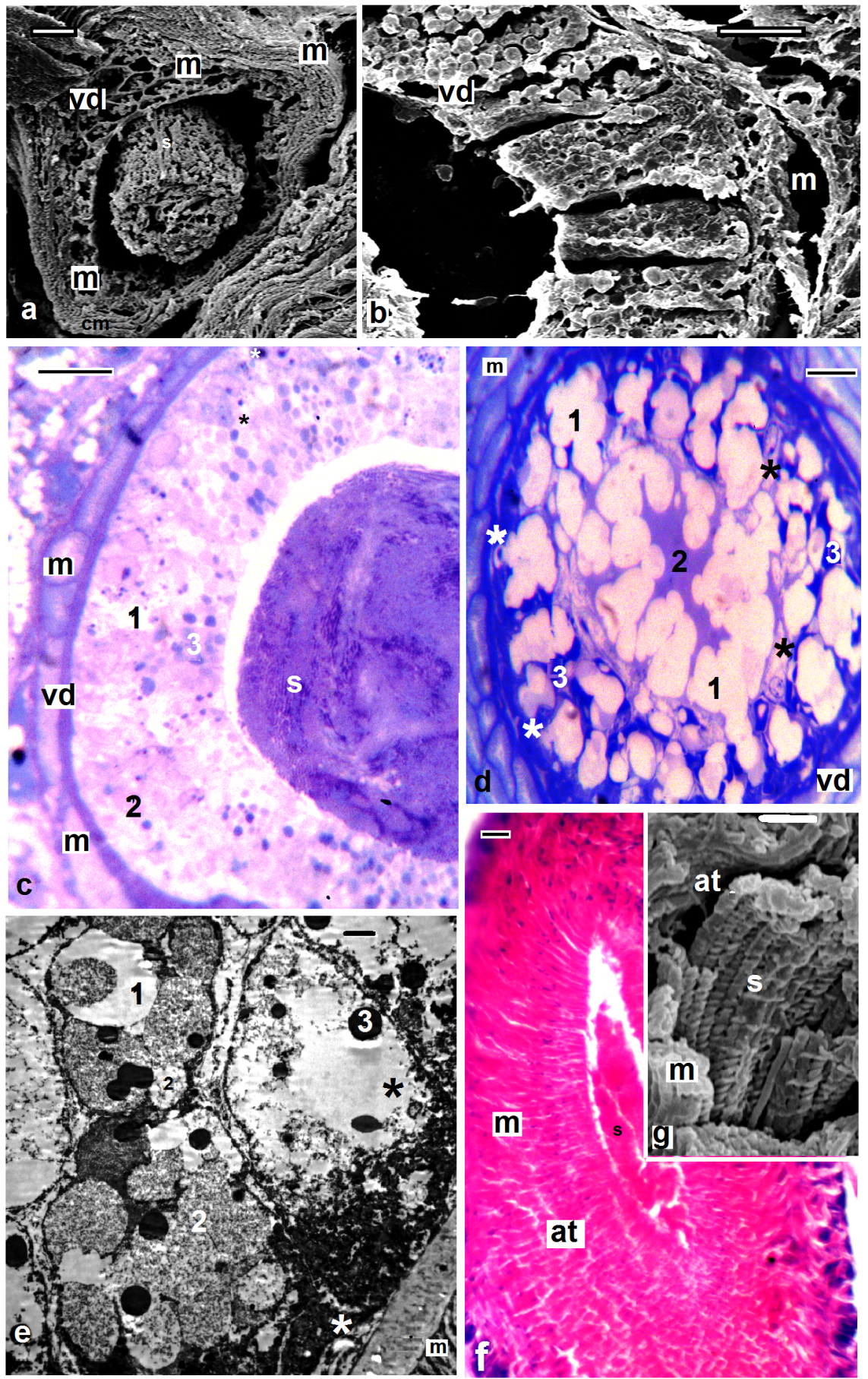
- Scanning electron microscopy (Figs. 1a, 2a,3a) and serial paraffin sections in testisacs of H. stagnalis (Figs. 1b,c) and S. delicata (Fig. 2b) revealed sequence of spermatogenic stages within the testisacs. Spermatogonia appeared in transmission electron microscopy (Fig. 1d) as large and polygonal cells measuring 6.25 x 5.25 um (mean), with large nucleus occupying most of the cell volume and numerous mitochondria (Fig. 1d). Spermatogonia divided into two (Fig. 1e) hence four isogenic groups of cells, in these stages, the cytophore was invisible (Fig. 1c). An anucleate cytophore appeared and was very small in the stage of eight isogenic cells stage (Figs. 1c,f). These cells were attached to the cytophore (Figs. 2c-f, 3b-e, 4d) by narrow cytoplasmic bridges ("collars" in[9]; "bridges" in[10]; "zonulae collaris" in[11]). Spermatogenetic stages were formed by cell division of spermatogonia without cytokinesis. As the development proceeded, the isogenic cells (16, 32, 64, 128 and finally 256 cells; the spermatides) became smaller and smaller in size gradually and conversely for the cytophore which became larger and larger. Number of spermatids was different in various taxa: 64 in the branchiobdellid Branchiobdella pentodonta[12,13], 128 in most of oligochaete species[14], but 256 in some lumbriculids; acanthobdellid Acanthobdella peledina; the leech Hirudo medicinalis,[12], similar to the same number of spermatids reported here in both studied H. stagnalis and S. delicata. In the phreodrilid Astacopsidrilus spermatids were more than 400[15], but 512 cells in the leech Dina lineata[16]. Though the precise number was unknown for most taxa. The present TEM revealed the fine structure of the cytophore and the cytoplasmic bridges (Figs. 2c-g, 3b-f). Numerous mitochondria, web – shaped endoplasmic reticulum and U – shaped cisternae of Golgi complex (Figs. 2f,g, 3f). The cytophore was formed by a progressive accumulation of cytoplasm and organells during the first spermatogonial divisions.[17] summarized the functions of the cytophore as the following: 1)synchronization of germ cell development; 2) production of energy through the large number of mitochondria present; 3) selective intake of material and organells discharged during spermiogenesis; 4) synthesis of materials connected to the sperm morphogenesis. The current study showed that spermatogenesis in both Helobdella stagnalis and Salifa delicata began and completed in testis-sacs.Spermatogenesis in earthworms began in the testes but completed either in seminal vesicle, as in some megascolecid or in testis-sacs, or in both types of receptacle as in Amynthas[18]. Stage of 256 isogenic cells or the spermatids, which attached to, the cytophore, these cells decreased in size and elongated gradually when developed into the spermatozoa; a process termed "spermiogenesis".
3.2. Spermiogenesis
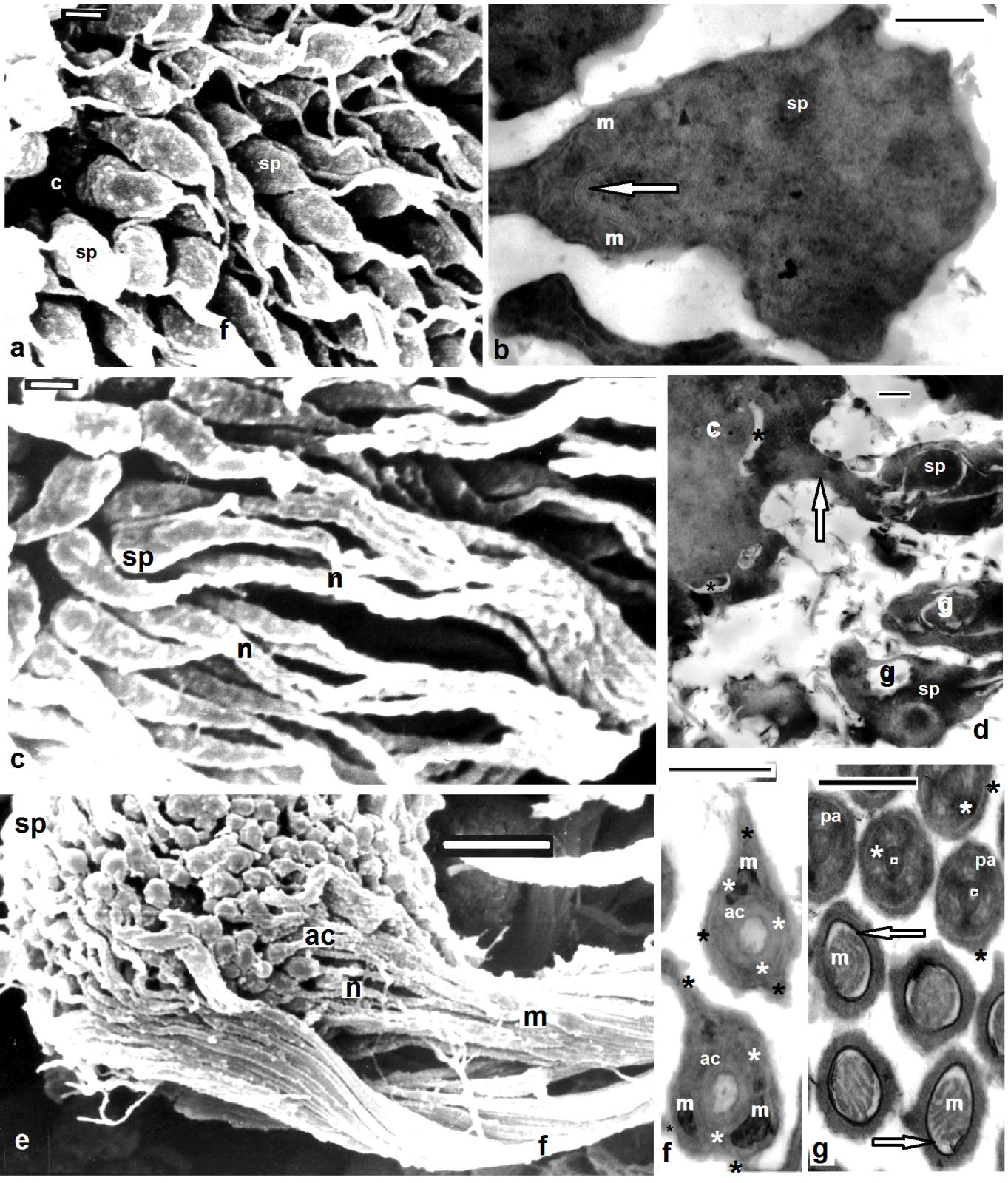
- Electron microscopy showed the sperm in both studied Helobdella stagnalis and Salifa delicata seemed as filiform with helicoid anterior half and smooth posterior part. Among leeches there is considerable consistency in the shape and maturation of the spermatozoon[2,16,19]. Morphological and histological examination revealed four distinguished regions; mentioned here (assuming the same order of formation) as the following: Flagellar, mitochondrial, nuclear and acrosomal regions (Figs. 3c, 4e, 5a-c, 6a-f). Flgellar region seemed by SEM and TEM as a long, smooth and flexible filament (Figs. 3d, 4a, 5b) which was formed at first. Flagellum in both current leeches appeared with 9+2 appearance as other euclitellates. The flagellum formed outstanding and peculiar solid central sheath with highly electron-dense structure (Figs. 5a,b) similar to the finding of[20]. SEM and TEM showed that the end piece of flagellum looked thinner than the anterior part (Fig. 6g). Mitochondrial region is located next to the flagellar region. Its external morphology was a simple helix (Fig. 3b). As it extended toward the nuclear region the helix became simply appeared and the current data revealed that the single spiral elongated mitochondrion of the coming spermatozoon was formed by the fusion and combination of mitochondria of the spermatid (Fig. 4b). The final number of mitochondria was reached through the migration of the extra mitochondria towards the cytophore in the, oligochaete Microchaetus pentheri,[21], or their fusion as in the oligochaete Homogaster redii[22] and Acanthobdella peledina[23], while the in leech Piscicola geometra occurred by the combination of both processes[24]. Numbers of mitochondria in the mature sperms were different within euclitellates; seven to four mitochondria were reported in Acanthobdella peledina, Branchiodebllidans whereas only one in leeches and Ancanthodbella[16,25]. TEM revealed the electron dense sheath surrounding the cristae of the mitochondrion (Figs. 4g, 5b). Nuclear region was made up by two fibers giving the helicoid appearance, in which the double helix was very compressed (Figs. 3c, 4c, 6d, h). Nuclear region seemed with a uniform appearance for most of its length. Transverse sections in TEM revealed trilobed twisted nucleus in both H. stagnalis (Fig. 5d) and S. delicata (Fig. 6g), agreed to the finding of[19] in the piscicolid leech Piscicola geometra. Nuclear region in Glossiphonia complanata appeared as a highly twisted thread of variable thickness[25], or a highly complex twisted column in Batracobdella paludosa[26]; Haementeria depressa[5]; Haemiclepsis marginata[25]. The acrosome; the most complicated structure in the spermatozoon appeared as long corkscrew– shaped. Externally, SEM revealed both anterior and posterior regions constituted a double helix of two elements with different thickness; a thin fiber around another thick. The thin fiber coils appeared spaced anteriorly, but convergent posteriorly till reaching the nuclear region (Figs. 6e,f). TEM detected an anterior extension of the tube; the anterior thin pointed acrosome and a wide acrosome rod; the posterior acrosome (Figs. 4f, 5a, 6e-i) with same diameter along its length. Here the acrosome tube appearedcorkscrew–shaped as other hirudineans[19,25], while straight in oligochaetes[27] and absent in polychaetes[28]. After spermiogenesis the produced sperms were collected together in bundles by secretions from the vasa differentia, passed to the atrium and gonopore to fertilize the recipient partner.
3.3. Sperm Transfer
4. Conclusions
- Spermatogenesis in both H. stagnalis and S. delicata began and completed in testis-sacs. Spermatozoa of both studied leeches appeared as filiform, elongated with four distinguished regions; a complicated corkscrew- shaped acrosome, a complex helical nucleus, a single mitochondrion surrounded by dense material and the flagellum which formed outstanding and peculiar solid central sheath with highly electron-dense structure. In S. delicata sperm transfer occurred by direct insemination, through copulation, but in H. stagnalis by dermal impregnation through the cuticle of the recipient mate.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML