-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
p-ISSN: 2325-002X e-ISSN: 2325-0038
2013; 3(1): 23-30
doi:10.5923/j.zoology.20130301.04
Assessment of the Immune Status and DNA Damage in Goats with Experimentally- Induced Hypocuprosis
Heba M. El-khaiat1, Mohamed M. Ghanem1, Hala A. Abou-Zeina2, Yassein M. Abd El-Raof1, Hossam M. El-Attar1, Sekena H. Abd El-Aziem3
1Animal Medicine Department, Faculty of Vet. Medicine, Benha University
2Parasitology and animal diseases department, Vet. Research Division, National Research Centre (NRC), Cairo, Egypt
3Cell Biology Department, Genetic Engineering and Biotechnology Division, National Research Centre (NRC), Cairo, Egypt
Correspondence to: Heba M. El-khaiat, Animal Medicine Department, Faculty of Vet. Medicine, Benha University.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
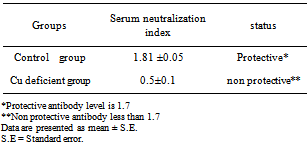
This study was carried out on 16 adult castrated male Baladi goats with age ranged from 1-1.5 years old and weight range of 19±0.82 kg to investigate the biological role of copper deficiency on the modification of immune status and induction of DNA damage. They were randomized into two groups. The first group (six goats) were apparently healthy and kept as control group, whereas the second group (ten goats) were subjected to experimental induction of secondary copper deficiency by dietary addition of Molybdenum ( MO; 10- 40 mg/kg DM) and Sulpher (S; 1.5-3 g / Kg dry matter) daily for 24 weeks. Blood samples were collected without anti-coagulant every six weeks for determination of serum copper and ceruloplasmin activity. Heparinized blood samples were used for assessment of immune status, DNA damage and erythrocyte superoxide dismutase (SOD). Results showed significant decrease (P<0.05) in serum copper, ceruloplasmin and erythrocyte SOD activity starting from 6 weeks to 24 weeks after addition of Mo and S. Goats with experimentally-induced copper deficiency have low serum neutralization antibody index (0.5±0.1) against inactivated rift valley fever vaccine which did not reach the protective antibody level (1.7) compared to that of the apparently healthy control group (1.81 ±0.05) which exceeded the protective value. Lymphocyte blastogenesis response of copper deficient goats was decreased although non significantly in comparison with the apparently healthy control group. The results also showed that copper deficiency caused marked increase in the % of DNA fragmentation of blood cells in goats with experimentally-induced copper deficiency compared to the apparently healthy control group. Goats with experimentally-induced copper deficiency have DNA fragmentation as detected by gel electrophoresis and the DNA ladder represented a series of fragments that is multiples of 180–200 bp. Our findings suggest a significant role of copper deficiency in modulation of immune status and induction of DNA damage and cell apoptosis in goats. Hence, copper level should be strictly considered during formulation of rations in farm animal production practice.
Keywords: Ceruloplasmin, DNA Damage, Hypocuperosis, Goats, Immunity - Lymphocyte Blastogenesis, Molybdenum, Sulpher, Superoxide Dismutase
Cite this paper: Heba M. El-khaiat, Mohamed M. Ghanem, Hala A. Abou-Zeina, Yassein M. Abd El-Raof, Hossam M. El-Attar, Sekena H. Abd El-Aziem, Assessment of the Immune Status and DNA Damage in Goats with Experimentally- Induced Hypocuprosis, Research in Zoology, Vol. 3 No. 1, 2013, pp. 23-30. doi: 10.5923/j.zoology.20130301.04.
1. Introduction
- Copper (Cu) deficiency in ruminants is a common worldwide mineral insufficiency problem[1] which can occur as a primary deficiency where Cu intake is inadequate, or as a secondary deficiency, where by other factors in the diet interfere with the absorption or metabolism of Cu e.g. molybdenum ( Mo) and sulfur (S)[2]. In the rumen Mo and S form thiomolybdates which bind with high affinity to dietary Cu in addition to antagonize Cu metabolism by decreasing absorption, increasing biliary excretion of Cu, and chelating Cu from metalloenzymes[3]. Copper deficiency is associated with impairment of number of metabolic processes includingimmune functions[4].Cu deficiency results in decreased humoral and cell-mediated immunity, as well as decreased nonspecific immunity regulated by phagocytic cells such as macrophages and neutrophils. Impairment of immune function may be highly correlated with an increased incidence of infection and higher mortality rates observed in copper-deficient animals[5].Two copper-dependent enzymes, ceruloplasmin (Cp) and superoxide dismutase (SOD), exhibit anti-inflammatory activity and play critical roles in the prevention of oxidative tissue damage resulting from infection and inflammation[6]. Ceruloplasmin may play an important role in the regulation of Cu transport to sites of inflammation for protection against tissue damage[5].Copper plays an important supporting role in preventing oxidative damage to critical cellular components. It is often assumed that failure to provide cells and tissues with an adequate supply of copper increase their susceptibility to oxidative stress. Moreover, Normal levels of copper must be present in cells to maintain the structural integrity of DNA during oxidative stress. Therefore, copper deficiency promotes oxidative DNA damage[7. Therefore, this study has the following aims:-1. Experimental induction of copper deficiency in goats2. Studying the effect of copper deficiency on the antioxidant activity of enzymes such as ceruloplasmin and superoxide dismutase.3. Evaluation of cellular and humeral mediated immune status in experimentally- induced secondary copper deficiency in goats.4. Monitoring the molecular changes (as being detected by DNA fragmentation occurring in the nuclei of hypocupermic goats.
2. Materials and Methods
- AnimalsA total number of 16 clinically healthy adult castrated Baladi male goats were used in this experiment. Their age and L.B.weight ranged from 1-1.5 years and 19±0.82 Kg respectively. The animals were placed in good hygienically well ventilated stable and kept under the same environmental, nutritional and hygienic conditions throughout the period of the experiment. Ration offered to the animals was basically composed of: 50% yellow corn, 25% cotton seed cake and 17% wheat bran. Additionally, the animals were supplemented with seasonal green fodders essentially Alfa Alfa (Green Barseem) in winter Straw) were added at nights while fresh drinking water was offered ad lib. They were randomized allotted into 2 groups: Group1: Included six goats that were kept as a control group. Group2: Included ten goats that subjected to experimental induction of copper deficiency by addition of 10- 40 mg Mo /kg DM and 1.5-3 g S / Kg DM daily onto the ration for about 24 weeks according to[8] with some relevant modification. Blood samplesBlood samples were collected (at 0, 6, 12 and 24weeks of the experiment) from the jugular vein according to[2]. Heparinized blood samples were collected for determination of erythrocytic SOD activity in the lysate and cellular immunity. Erythrocyte lysate was obtained from centrifugation of the blood sample at 1000rpm for 10 minutes for separation of plasma. The erythrocytes were lysed in 4 times its volume of ice cold HPLC grade water Centrifuged at 10,000 for 15 minutes at 4 °C. Erythrocytes lysate was then collected and stored at -80 ◦C until the assigned and time of analysis according to[9]. Plain vacutainer tubes were used for obtaining a clear non-hemolyzed serum by centrifugation of the blood sample at 3000 rpm. for 5 minutes. Sera were used for determination of serum copper and ceruloplasmin activity. Serum samples for serum neutralization test (SNT) were collected 8 weeks after vaccination of the animals with inactivated RVF vaccine to determine the antibody titres. Heparinized blood samples were collected at the end of the experiment for monitoring the molecular changes by detection of DNA fragmentation.Biochemical analysesCopper concentrations were determined in serum using Flame Atomic Absorption Spectrophotometer according to[10]. Erythrocyte superoxide dismutase activity (SOD) was assayed by using commercially available diagnostic kit (Bio-diagnostic Co. Egypt) .This assay relies on the ability of the enzyme to inhibit the phenazine methosulphate-mediated reduction of nitro blue tetrazolium dye. Erythrocyte SOD activity was carried out according to[9]. Ceruloplasmin oxidase activity was assayed according to the method described by[11].Evaluation of immune response Heparinized blood samples for lymphocyte blastogenesis assay were collected at 12thweek of the experiment during the stage of subclinical copper deficiency. After 4 weeks (at 16th week of the experiment) other blood samples were collected for separation of unstimulated lymphocytes for lymphocyte blastogenesis assay. A- Evaluation of cell mediated immune response by lymphocyte blastogenesis assay using XTT kitsThe lymphocyte blastogenesis assay was based on the cleavage of the tetrazolium salts (3-[4, 5-dimethyl thiazol-2yl]-2, 5-diphenyl tetrazolium bromide) into a blue coloured product (formzan) by the mitochondria enzyme succinate dehydrogenase[12] is very useful for assaying the cell survival and proliferation. This conversion takes place in the living cells and the amount of formazan produced is proportional to the number of cells present. The assay was performed in 2 consecutive stages. During the first stage, separation of lymphocytes was applied according to[13]. Heparinized blood samples were collected from the jugular vein in vacuum tube under complete aseptic condition. Whole blood diluted (1:2) with sterile PBS, layered on Ficol-Hypaque, and centrifuged at 40xg for 30 minutes. The band at the interface between the plasma and the ficol layers was aspirated, washed three times with serum free RPMI-1640 MEDIUM, and resuspended in RPMI-1640 supplemented with 10% foetal calf serum. Viability of separated lymphocytes was determined according to[14]. Cell number and viability was determined using the trypan blue exclusion test. The cell diluted in 0.1% trypan blue, loaded on haemocytometer and the cells counted in 32 small squares. The cell number per ml calculated according to the following formula:
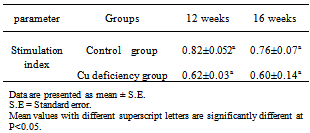
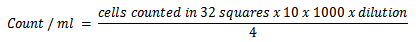 According to the viability cell count the viable lymphocytes were adjusted to a concentration of 5 x 106 cells/ ml suspended in 1 ml RPMI containing 10% FCS. Secondly, the test was conducted according to[15]. Briefly, cells were plated into 96-well tissue culture plates (Nunc, Denmark). 100µl of growth media were added to each well. In general, cells were seeded at densities between 5000 and 10,000 cells per well since they reached optimal population densities within 24 to 96 hours in CO2 incubator at 37º c. The final volume of tissue culture medium in each well was 0.1ml and the medium contained up to 10% fetal calf serum. During the second stage, The XTT reagent solution and the activation solution were defrosted immediately prior to use in a 37°C. To prepare a reaction solution sufficient for one plate (96 wells); 0.1ml activation solution was added to 5ml XTT reagent. Twenty five μL of the activated XTT solution were Added to each well and the plate were incubated the plate in a CO2 incubator at 37º C for 4 hours. The plate was shacked gently to evenly distribute the dye in the wells. The absorbance of the samples was measured with a spectrophotometer (ELISA reader) at a wavelength of 450-500 nm.B- Evaluation of humeral immune response by Serum Neutralization Test (SNT) against inactivated Rift valley fever vaccineInactivated Rift valley vaccine was obtained from Vet. Ser. and Vacc. Res. Inst., Cairo, Egypt. It was used for vaccination of all goats (control and hypocupermic goats) according the manufacturer's instructions (1 ml/ animal via the subcutenous route). Goats were vaccinated at 16th week of the experiment and blood samples without anticoagulant were collected 8 week (2months) post vaccination according to[16] who reported that the period of higher protective antibody titer (1.7) against inactivated RVF vaccine was 2 months. Serum Neutralization Test was carried out to determine antibody titer of rift valley fever according to[17].The DNA Fragmentation (Apoptosis) AssayApoptosis, or physiological cell death, can be distinguished from necrosis on the basis of a series of morphological and biochemical parameters. In general, in order to describe a death event as apoptosis, more than one parameter should be evaluated. Among these parameters, DNA fragmentation is very typical of the apoptotic process, with generation within the nucleus of a series of multiples of a 180 bp subunit, through the action of a Ca++/Mg++-dependent endonuclease that cleaves DNA in the linker region between nucleosome cores.This method is based on the notion that extensively fragmented double-stranded DNA can be separated from chromosomal DNA upon centrifugal sedimentation. The protocol includes the lysis of cells and the release of nuclear DNA, a centrifugation step with the generation of two fractions (intact and fragmented DNA), precipitation of DNA, hydrolysis and colorimetrical quantitation upon staining with diphenylamine (DPA), which binds to deoxyribose. Apoptotic changes in animals were evaluated colorimetrically by DNA stained with DPA and by monitoring fragmented DNA using agarose gel electrophoresis according to the procedure of[18] with some modifications, the excitation wavelength of 600 nm is the optimal one, but wavelengths from 560 to 620 can, however, be used as well. The experimental procedure for detecting DNA fragmentation from blood proceeded as following:• DNA extraction from the fragmented blood clot mass obtained from the trimming of the clot followed the standard procedures typically used for DNA extraction from whole blood. • Blood cells were lysed with 1 mL of Tris buffer 1 (10 mmol/L Tris-HCl, pH 8.0, 10 mmol/L KCl, 10 mmol/L MgCl2, 2 mmol/L EDTA, pH 8.0, and 25 mL/L Triton X-100) and centrifuged at 10000 rpm for 15 min at 4˚C. The supernatants (SN) containing small DNA fragments were separated one half of the volume was used for gel electrophoresis, the other half of SN and the pellet containing large pieces of DNA were used for quantification of fragmented DNA by the Diphenylamine (DPA) assay. • One- half of supernatant of each sample was treated with equal volume of absolute isopropyl alcohol and 0.5 M NaCl to precipitate DNA. These samples were then kept at -20˚C over night and centrifuged at 10000 rpm for 15 min at 4˚C. • The supernatants were discarded and the pellets were then washed with 200 µl of 70% ethanol and then centrifuged at 10000 rpm for 10 min at 4˚C. • The supernatants were discarded and the pellets were then washed with 200ul of 70% ethanol centrifuged at 10000 rpm for 10 min at 4˚C. • Extracted DNA was re-suspended in 50 µl Tris-EDTA buffer (10 mmol/L Tris -base and 1mmol/L EDTA, pH8.0) the samples were incubated at 37˚C for 20 min. 12 µl of sample mixed with 3 µl of bromophenol blue and then electrophoresed on 1% agarose gel containing 0.71 µg/ml ethidium bromide. At the end of runs, gel was examined using UV transilluminationThe Diphenylamine (DPA) assay:The Diphenylamine (DPA) assay was modified by[18]. Briefly, 200 µl of 0.5 M perchloric acid was added to pellets containing native DNA and 200 µl of 5.5 M perchloric acid was added to the other half of supernatant of samples to reach the final concentration to 0.5 M. Then these samples incubated in water bath at 90˚C for 20 min then centrifuged at 1500 rpm for 10 min at 4˚ C. Supernatant was transferred to clean glass tubes containing 800 µl of DPA working solution (0.088 M DPA, 98% glacial acetic acid, 1.5% sulfuric acid and 0.5% acetaldehyde). The samples were kept at room temperature for 24 hour. The coloremetric reaction was then measured spectrophotometrically at 600 nm.The percentage of DNA fragmentation was expressed by the following formula:
According to the viability cell count the viable lymphocytes were adjusted to a concentration of 5 x 106 cells/ ml suspended in 1 ml RPMI containing 10% FCS. Secondly, the test was conducted according to[15]. Briefly, cells were plated into 96-well tissue culture plates (Nunc, Denmark). 100µl of growth media were added to each well. In general, cells were seeded at densities between 5000 and 10,000 cells per well since they reached optimal population densities within 24 to 96 hours in CO2 incubator at 37º c. The final volume of tissue culture medium in each well was 0.1ml and the medium contained up to 10% fetal calf serum. During the second stage, The XTT reagent solution and the activation solution were defrosted immediately prior to use in a 37°C. To prepare a reaction solution sufficient for one plate (96 wells); 0.1ml activation solution was added to 5ml XTT reagent. Twenty five μL of the activated XTT solution were Added to each well and the plate were incubated the plate in a CO2 incubator at 37º C for 4 hours. The plate was shacked gently to evenly distribute the dye in the wells. The absorbance of the samples was measured with a spectrophotometer (ELISA reader) at a wavelength of 450-500 nm.B- Evaluation of humeral immune response by Serum Neutralization Test (SNT) against inactivated Rift valley fever vaccineInactivated Rift valley vaccine was obtained from Vet. Ser. and Vacc. Res. Inst., Cairo, Egypt. It was used for vaccination of all goats (control and hypocupermic goats) according the manufacturer's instructions (1 ml/ animal via the subcutenous route). Goats were vaccinated at 16th week of the experiment and blood samples without anticoagulant were collected 8 week (2months) post vaccination according to[16] who reported that the period of higher protective antibody titer (1.7) against inactivated RVF vaccine was 2 months. Serum Neutralization Test was carried out to determine antibody titer of rift valley fever according to[17].The DNA Fragmentation (Apoptosis) AssayApoptosis, or physiological cell death, can be distinguished from necrosis on the basis of a series of morphological and biochemical parameters. In general, in order to describe a death event as apoptosis, more than one parameter should be evaluated. Among these parameters, DNA fragmentation is very typical of the apoptotic process, with generation within the nucleus of a series of multiples of a 180 bp subunit, through the action of a Ca++/Mg++-dependent endonuclease that cleaves DNA in the linker region between nucleosome cores.This method is based on the notion that extensively fragmented double-stranded DNA can be separated from chromosomal DNA upon centrifugal sedimentation. The protocol includes the lysis of cells and the release of nuclear DNA, a centrifugation step with the generation of two fractions (intact and fragmented DNA), precipitation of DNA, hydrolysis and colorimetrical quantitation upon staining with diphenylamine (DPA), which binds to deoxyribose. Apoptotic changes in animals were evaluated colorimetrically by DNA stained with DPA and by monitoring fragmented DNA using agarose gel electrophoresis according to the procedure of[18] with some modifications, the excitation wavelength of 600 nm is the optimal one, but wavelengths from 560 to 620 can, however, be used as well. The experimental procedure for detecting DNA fragmentation from blood proceeded as following:• DNA extraction from the fragmented blood clot mass obtained from the trimming of the clot followed the standard procedures typically used for DNA extraction from whole blood. • Blood cells were lysed with 1 mL of Tris buffer 1 (10 mmol/L Tris-HCl, pH 8.0, 10 mmol/L KCl, 10 mmol/L MgCl2, 2 mmol/L EDTA, pH 8.0, and 25 mL/L Triton X-100) and centrifuged at 10000 rpm for 15 min at 4˚C. The supernatants (SN) containing small DNA fragments were separated one half of the volume was used for gel electrophoresis, the other half of SN and the pellet containing large pieces of DNA were used for quantification of fragmented DNA by the Diphenylamine (DPA) assay. • One- half of supernatant of each sample was treated with equal volume of absolute isopropyl alcohol and 0.5 M NaCl to precipitate DNA. These samples were then kept at -20˚C over night and centrifuged at 10000 rpm for 15 min at 4˚C. • The supernatants were discarded and the pellets were then washed with 200 µl of 70% ethanol and then centrifuged at 10000 rpm for 10 min at 4˚C. • The supernatants were discarded and the pellets were then washed with 200ul of 70% ethanol centrifuged at 10000 rpm for 10 min at 4˚C. • Extracted DNA was re-suspended in 50 µl Tris-EDTA buffer (10 mmol/L Tris -base and 1mmol/L EDTA, pH8.0) the samples were incubated at 37˚C for 20 min. 12 µl of sample mixed with 3 µl of bromophenol blue and then electrophoresed on 1% agarose gel containing 0.71 µg/ml ethidium bromide. At the end of runs, gel was examined using UV transilluminationThe Diphenylamine (DPA) assay:The Diphenylamine (DPA) assay was modified by[18]. Briefly, 200 µl of 0.5 M perchloric acid was added to pellets containing native DNA and 200 µl of 5.5 M perchloric acid was added to the other half of supernatant of samples to reach the final concentration to 0.5 M. Then these samples incubated in water bath at 90˚C for 20 min then centrifuged at 1500 rpm for 10 min at 4˚ C. Supernatant was transferred to clean glass tubes containing 800 µl of DPA working solution (0.088 M DPA, 98% glacial acetic acid, 1.5% sulfuric acid and 0.5% acetaldehyde). The samples were kept at room temperature for 24 hour. The coloremetric reaction was then measured spectrophotometrically at 600 nm.The percentage of DNA fragmentation was expressed by the following formula: 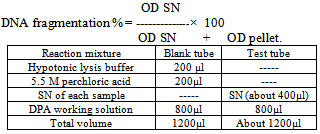 Statistical analysisStatistical analysis of the results was carried out using one-way ANOVA test according to[19]. Data were represented as means ± standard error (SE) of the means. The means were considered as statistically significant when P<0.05.
Statistical analysisStatistical analysis of the results was carried out using one-way ANOVA test according to[19]. Data were represented as means ± standard error (SE) of the means. The means were considered as statistically significant when P<0.05.3. Results and Discussion
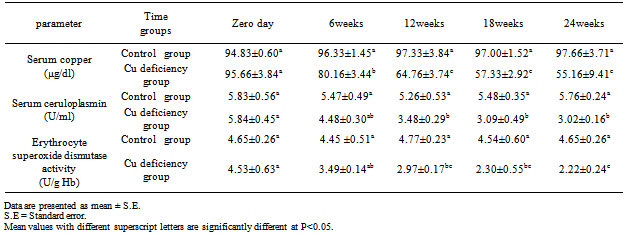
- The results showed that addition of 10-40 mg Mo /kg DM and 1.5- 3 gm S /kg DM to the experimental ration of the goat succeeded to induce a secondary copper deficiency status in goats. This result coincided well with that obtained by[8],[20],[21] and[22] who added 10 mg Mo and 3 gm S /kg dry matter to the compound ration of lambs for induction of secondary copper deficiency. The specific effect of molybdenum in producing clinical copper deficiency symptoms can be detected when Mo combined with S in the rumen to form thiomolybdates which bind with high affinity to dietary Cu in addition to antagonizing Cu metabolism by decreasing absorption, increasing biliary excretion of Cu, and chelating Cu from metalloenzymes[3]. The clinical signs of secondary copper deficiency are likely to be from formation of thiomolybdate (MoS4) in the body[23].Careful clinical examination of experimentally induced hypocupermic goats revealed changes in hair color and texture at the 9th week of the experiment. Later on, the fine hair becomes limp, glossy and steely appearance. Moreover, the black hair showed depigmentation (Fig.1) and became easily to be detached. The clinical signs of hair changes and depigmentation became more obvious at the end of the experimental period. Hair depigmentation associated with Cu deficiency in cattle may be attributed to reduction in the activity of tyrosinase which is Cu-dependant enzyme required for melanin synthesis[3]. Hypocupermic goats showed paleness of the conjunctival mucous membrane (Fig.2) from the beginning of the 18th week of the experiment which considered as a fair sign of anaemia. This result was similar to those obtained by[1],[4],[22] and[24]. Copper deficiency has been found to cause anemia due to disturbances in iron metabolism resulting in sequestration of iron by the liver due to decreased plasma ceruloplasmin activity which is involved in the mobilization of tissue iron[5]. At the beginning of the 21st week of the experiment, clinical examination showed hyperthesia, nervous manifestation and stiffness in gait. These results coincided well with those of a previous study[25] that explained the defects which affect the skeleton of Cu-deficient animals as being biochemically related to disorder cross-linking of connective tissue proteins caused by a deficiency of lysyl oxidase. Disorders of the nervous system have been linked to a lack of cuproenzymes dopamine-ß- hydroxylase involved in the conversion of dopamine to norepinephrine[6].[24] explained that Cu is necessary for formation of myelin sheath, thus Cu deficient animal exhibit nervous disorders. Emaciation and loss of body condition (Fig.3) became more obvious signs at the 24th week of the experiment. This result matched well with those of[1],[26] and[27]. The signs of growth retardation in copper deficient animals are related to reduction in the activity of cuproenzymes such as cytochrome-c-oxidase which is important for energy production[28].The data demonstrated in table (1) show that the mean values of serum copper in experimentally induced copper deficient animals were significantly (P<0.05) low at 6weeks as compared with apparently healthy control goats. This result may be attributed to the presence of potent Cu antagonists such as Mo and S which form thiomolybdates in the rumen which bind with high affinity to dietary Cu in addition to antagonizing Cu metabolism by decreasing absorption, increasing biliary excretion of Cu, and chelating Cu from metalloenzymes[3],[29] and[30].
|
|
|
 | Figure 1. Hypocupremic goat showed hair depigmentation and steely appearance |
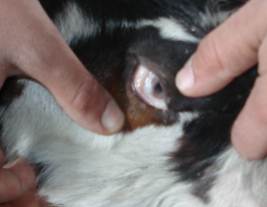 | Figure 2. Hypocupremic goats showed paleness of conjunctival mucous membrane |
 | Figure 3. Hypocupremic goat showed emaciation and loss of body condition |
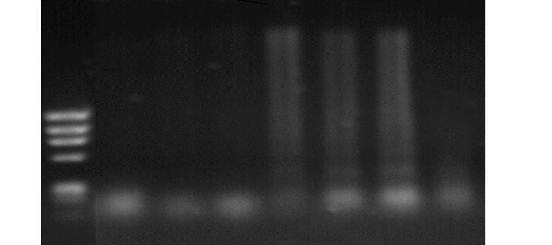 | Figure 4. Agarose gel electrophoretic pattern of DNA isolated from blood of goats showing fragmentation of DNA in samples with cupper deficiency. Lane M: marker (ô x 174 DNA); Lane I, 2 and 3: control group; Lane 3, 4, 5, 6 and 7: copper deficiency group |
ACKNOWLEDGMENTS
- This research was conducted in cooperation with the National Research Center as a part of a project: entitled ‘Improvement of general health condition and immune status of small ruminants using antioxidants’ (The 9th research plan, 2010-2013, No. 9040203)
References
| [1] | Hansen, S. L.; Ashwell, M. S.; Legleiter, L. R.; Fry, R. S.; Lloyd, K. E.; and Spears, J.W.: The addition of high manganese to a copper-deficient diet further depresses copper status and growth of cattle. British Journal of Nutrition .101, 1068–1078,2009. |
| [2] | Radostits, O. M.; Gay, C. C.; Hinchcliff, K. W. and Constable, P.D.: Veterinary Medicine, 10th Ed. W. B. Saunders Company Ltd, London, New York, Philadelphia, san Francisco, St Louis, Sydney.1707-1722,2007. |
| [3] | Fry, R. S.: Dietary and Genetic Effects on Cellular Copper Homeostasis in Bovine and Porcine Tissues. Ph.D. Thesis, Faculty of North Carolina, State University,2011. |
| [4] | Sharma, M. C.; Joshi, C. and Das G.: Therapeutic management of copper deficiency in buffalo heifers: Impact on immune function. Vet. Res. Commun. 32:49–63, 2008. |
| [5] | Stable, J. R.; Spears, J. W. and Brown, T. T.: Effect of copper deficiency on tissue, blood characteristics and immune function of calves challenged with infectious bovine rhinotracheitis virus and pasteurella haemolytica. J. Animal. Sci., 71:1247-1255,1993. |
| [6] | Cerone, S. I.; Sansinanea, A. S.; Streitenberger, S. A.; Garcia, M. C.and Auza, N. J.: Cytochrome-c-oxidase, Cu-Zn-superoxide dismutase, and ceruloplasmin activities in copper-deficient bovines. Biological Trace Element Research 73, 3, 269-278,2000. |
| [7] | Pan,Y.J. and Loo,G.: Effect of copper deficiency on oxidative DNA damage in Jurkat T-lymphocytes. Free Radic. Biol. Med., 28, 824—830,2000. |
| [8] | Moeini, M. M.; Souri, M. and Nooriyan, E.: Effect of molybdenum and sulpher on copper status and Mohair quality in Merghoze goat. Pakistan Journal of biological sciences, 11 (10):1375-1379,2008. |
| [9] | Nishikimi, M. Appaji, N. and Yagi, K.: The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commum., 46: 849-854,1972. |
| [10] | Fernandez, F.J. and Kahan, H.L.: Clinical Methods for Atomic Absorption Spectroscopy. Clinical Chemistry News, 1: 3-24, 1971. |
| [11] | Schosinsky, K. H.; Lehmann, H. P. and Beeler, M. F. (1974): Measurement of Ceruloplasmin from Its Oxidase Activity in Serum by Use of O-Dianisidine Dihydrochlorid. Clinical chemistry, Vol. 20, No. 12.1556-1563,1974. |
| [12] | Slater, T. F., Sawyer, B. and Strauli, U. (1963): Rapid coloremetric assay for cell growth and survival Modification of the tetrazolium dye procedure giving improved sensitivity and reliability. J. immunol. Meth, 89: 271- 277, 1963. |
| [13] | Lee, L. F.: Proliferative response of chicken B and T lymphocyte to mitogens. Vet. Med.; 15:44-52,1984 |
| [14] | Mayer, S. P.; Ritts, G. D. and Johnson, D. R.: Phytohaemagglutinin induced leukocyte blastogenesis in normal and avian leucosis virus infection in chicken cells. Immunol; 27, 140-146,1974 |
| [15] | Scudiero, D. A.; Shoemaker, R. H.; Paull, K. D.; Monks, A.; Tierney, S.; Nofziger, T. H.; Currens, M. J.; Seniff, D. and Boyd, M. R.: Evaluation of a Soluble Tetrazolium/Formazan Assay for Cell Growth and Drug Sensitivity in Culture Using Human and Other Tumor Cell Lines. Cancer Res.; 48:4827-4833,1988 |
| [16] | Marawan, M. A.: Some studies on Rift Valley fever. M.V.Sc, Thesis, Fac. Vet. Med., Benha University, Moshtohor,2012. |
| [17] | OIE Manual: Manual for standards for diagnostic tests and vaccines,1996. |
| [18] | Salazar LA, Hirata MH, Cavalli SA, Machado MO, Hirata RD.: Optimized procedure for DNA isolation from fresh and cryopreserved clotted human blood useful in clinical molecular testing. Clin Chem; 44:1748-1750,1998. |
| [19] | Bailey, R. A.: Design of Comparative Experiments. Cambridge University Press, 2008. |
| [20] | Suttle, N. F.: The interactions between copper, molybdenum, and sulphur in ruminant nutrition. Annu. Rev. Nutr. 11:121-140,1991. |
| [21] | Cerone, S. L.; Sansinanea, A. and Auza, N. : Copper deficiency alters the immune response of bovine DVM Nutrition Research, Vol. 15. No. 9. pp. 133-134,1995. |
| [22] | Mobarak, M.G.: Correlation between some serum trace elements and resistance among sheep. Ph.D. Thesis, Fac. Vet. Med., Zagaig University, Benha Branch, Moshtohor, 1998. |
| [23] | Sanjabi, M. R. Moeini, M. M. and Telfer, S. B.:Thiomolybdate – the major factor on clinical copper deficiency. Acta vet. scand. Suppl. 98, 258-260,2003. |
| [24] | Soetan, K. O.; Olaiya, C. O. and Oyewole, O. E.: The importance of mineral elements for humans, domestic animals and plants: A review. African Journal of Food Science Vol. 4(5) pp. 200-222, 2010. |
| [25] | Aupperle, H.; Schoon, H. A. and Frank, A. :Experimental copper deficiency, chromium deficiency and additional molybdenum supplementation in goats – pathological findings. Acta Vet. Scand. 42, 311-32, 2001. |
| [26] | Cerone, S. I.; Sansinanea, A. S.; Streitenberger, S. A.; Garcia, M.C. and Auza, N. J. : The effect of copper deficiency on the Peripheral blood cells of cattle. Veterinary Research Communications, 22 47-57,1998. |
| [27] | Gengelbach, G. P. and Spears, J. W.: Effects of Dietary Copper and Molybdenum on Copper Status, Cytokine Production, and Humeral Immune Response of Calves. J. Dairy Sci. 81:3286–3292,1998. |
| [28] | National Research Council: Nutrient requirements of beef cattle, 7th ed.Washington, DC:National Academy Press,1996. |
| [29] | Shalaby, H. A.; Younis, S. S. and Aly, M. A.: Role of the wool analysis in diagnosis of some nutritional deficiency diseases. Assiut Vet. Med. J. Vol. 56 No. 125,2010. |
| [30] | Vázquez-Armijo, J. F.; Rojo1, R.; Salem, A. Z. M.; López1, D.; Tinoco1, J. L.; González, A.; Pescador, N. and Domínguez-Vara, I. A.: Trace elements in sheep and goats reproduction: a review. Tropical and Subtropical Agroecosystems, 14: 1 – 13, 2011. |
| [31] | Naithani, V.; Singhal, A.K. and Chaudhary, M.: Comparative evaluation of Metal Chelating, Antioxidant and Free Radical Scavenging activity of TROIS and six products commonly used to control pain and inflammation associated with Arthritis. International Journal of Drug Development & Research, Vol. 3. Issue 4,2011. |
| [32] | Sharma, M.C.; Joshi, C.; Pathak, N. N. and Kaur, H. (2005): Copper status and enzyme, hormone, vitamin and immune function in heifers. Research in Veterinary Science.Vol. 79, Issue 2, Pp. 113-123,2005. |
| [33] | Picco, S.J., De Luca, J.C.; Mattioli, G. and Dulout, F.N.: DNA damage induced by copper deficiency in cattle assessed by the Comet assay. Mutation Research 498, 1–6,2001. |
| [34] | Ward, J. D.; Gengelbach, G. P.; and. Spears, J .W. (1997): The effects of copper deficiency with or without high dietary iron or molybdenum on immune function of cattle. J.Anim.Sci.75:1400–1408,1997. |
| [35] | Arthington, J.D.; Spell, A. R.; Corah, L.R. and Blecha, F.: Effect of molybdenum-induced copper deficiency on in vivo and in vitro measures of neutrophils chemotaxis both before and following an inflammatory stressor. J Anita Sci, 74: 22759-64,1996. |
| [36] | Bala, S.; Failla, M. L. and Lunney J. K. : Alteration in splenic lymphoid cell subsets and activation antigens in copper deficient rats. J. Nutr. 121:745,1991. |
| [37] | Picco S.J.; Abba1, M. C.; Mattioli, G. A.; Fazzio,L .E.; Rosa, D.; DeLuca1, J. C. and Dulout, F. N.: Association between copper deficiency and DNA damage in cattle. Mutagenesis Vol. 19 No. 6 PP. 453-456,2004. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML