-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
p-ISSN: 2325-002X e-ISSN: 2325-0038
2013; 3(1): 15-22
doi:10.5923/j.zoology.20130301.03
Acids Supplementation to Drinking Water and Their Effects on Japanese Quails Experimentally Challenged with Salmonella Enteritidis
Dalia Mansour Hamed1, Ahmed Mohamed Ahmed Hassan2
1Department of poultry and rabbit medicine, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt
2Department of Animal Hygiene & Zoonoses and Animal Behavior. Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt
Correspondence to: Dalia Mansour Hamed, Department of poultry and rabbit medicine, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Trials to in vitro evaluate organic and non-organic acids capacity in reducing microbial contamination of quail's drinking water and experimentally inhibit Salmonella Enteritidis (SE) culture growth as well as in vivo effect on microbial shedding and colonization in Japanese quail were conducted. For in vivo evaluation, a total of 200 one-day old Salmonella-free Japanese quails were assigned randomly into five groups (n= 40 in two replicates) as follow: Group I served as non-treated control received normal tap water, while groups II, III, IV and V received acetic acid (3 ml/L), acetic acid (0.5 ml/L), organic acids mixture (3 ml/L) and Hydrochloric acid (HCL) (0.5 ml/L), respectively in their drinking water till the end of experiment. Results revealed higher inhibitory capacity of organic acids as compared to HCL in vitro; whereas the best effect on reducing both drinking water bacterial count and inhibiting SE growth in culture medium was for group II followed by groups III and IV as compared to both groups I and V. In vivo, results revealed the same pattern on microbial colonization in both the gastrointestinal tract and internal organs (liver, spleen and reproductive organs). The lowest shedding count and colonization was observed in group II. Mortality and morbidity was highest in non-treated controls after experimental infection with SE followed by both groups III and IV. The highest protection was in group II. In conclusion, the study indicated higher in vitro antimicrobial capacity and in vivo protective role against microbial shedding and colonization of organic acids over in-organic acids. Higher level of drinking water acidification produced better results. Further studies are needed to evaluate tolerable levels of water acidification by birds and whether continues or interrupted acidifier’s administration in drinking water has more beneficial effects.
Keywords: Prebiotic, Acidifier, Japanese Quail, Salmonella Enteritidis, Colonization
Cite this paper: Dalia Mansour Hamed, Ahmed Mohamed Ahmed Hassan, Acids Supplementation to Drinking Water and Their Effects on Japanese Quails Experimentally Challenged with Salmonella Enteritidis, Research in Zoology, Vol. 3 No. 1, 2013, pp. 15-22. doi: 10.5923/j.zoology.20130301.03.
1. Introduction
- During the past 50 years, poultry industry has made great strides in several areas including nutrition, thereby maximizing the efficiency of growth performance and meat yield. Antibiotics using in animal feed for improving growth rate and feed efficiency, as well as for the prevention and treatment of diseases was a common practice (Luckstadt, 2005). However, improper usage increases the possibilities of antibiotic residue, the development of drug-resistant bacteria, and a reduction in the ability to cure these bacterial diseases in humans (Hays, 1986; Hawkey, 1998). Also increase animal's susceptibility to diseases and increase the hazards of transmitting such diseases to humans (Dibner, 2004; Luckstadt, 2005). Increased awareness of the potential problems associated with the use of antibiotics has stimulated research efforts to identify alternatives to their use as feed additives. Alternative strategies (including: organic acids and their salts, probiotics, prebiotics, enzymes, biogene additives (essential oils, herbal extracts etc.) were developed to cope with the removal of antibiotic as growth promoters (Bauermann, 2006; Gedek, 1999; Wald, 2004). Short chain organic acids (C1-C7) or their salts have been used for a long time because they prevent growth of molds and formation of mycotoxins (Gedek, 1999; Liu, 2001; Luckstadt, 2005). In recent years, there is an increasing trend in using organic acid and mixtures, as alternatives to antibiotic growth promoters due to their inhibiting activity on the growth and development of pathogens in animal feed and gastrointestinal tract (Wald, 2004 & Jovank et al. 2008). Each acid exhibit specific spectrum of antimicrobial activity, particularly Salmonella, is inhibited as early as in the seed sprouting stage. The required amount of acids, depend, in general, on the type of raw material, that is, on buffering capacity of the acids used (Luckstadt, 2005).Acidification of diets with weak organic acids have been reported to decrease colonization of pathogen and production of toxic metabolites, improved digestibility of protein, Ca, P, Mg, Zn and served as substrate in the intermediary metabolism (Kirchgessner and Roth, 1988; Veeramani et al. 2003). Hydrochloric acid is one of the main digestive fluids secreted in the stomach of birds and aid in feed digestion. Salmonella is a pathogenic organism and is not part of "normal flora". Salmonella is carried by, and causes disease manifested by irritation of the intestinal wall and decrease villi number and length which in turn impair nutrients absorption (Pelicano et al. 2005). Furthermore, it may compete with their host for nutrients and produce toxins, such as ammonia or amines, which damage the hosts liver (Krinke and Jamroz, 1996), ultimately resulting in more nutrients available for birds (Miles et al. 2006). Based on a previous study of Hassan et al. 2009, using different water supplements acetic acid superiority over both sodium bicarbonate or potassium chloride initiated the questions weather type of acid (organic or inorganic) or level of acidification would have different effects on the microbial colonization or effect on the microorganisms in vitro, so the main objective of this research was to compare the effect of organic and inorganic acidifiers in vitro followed by quantitative evaluation of their inhibiting effect on total bacterial count and on reducing shedding of Salmonella Enteritidis after experimental infection of Japanese quails.
2. Material and Methods
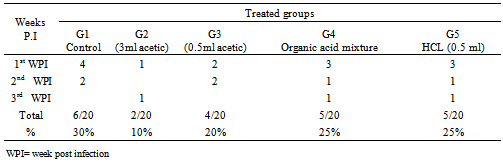
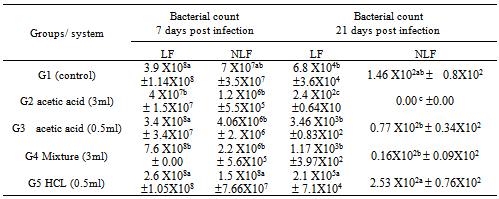
- A. In vitro evaluation of antimicrobial activity of different acidifiersThe antimicrobial activity of different acidifiers was evaluated by:1. Time correlated count in culture media according to modified method of Jackie and Mark (2005). Briefly, 1ml of 2X105 Salmonella Enteritidis was inculcated into 9ml tetrathionate broth. Different acidifier’s concentrations were added to different tubes by same concentrations that were applied in vivo (Tap water, 3ml.0.5ml acetic, organic acids mixture and HCL. Tubes then were incubated at 37oC for 1 hour, 2hours and 24 hours after each time interval absorbance was measured using a spectrophotometer. A standard curve was plotted for different absorbencies in control against bacterial count. Treated samples counts were recorded and inhibition percentages were calculated as correlated to their respective controls.2. Inhibitory capacity of different acidifiers in quail’s drinking water:Total bacterial count in drinking water treated with different acidifiers was evaluated as follow: By the end of the day after the birds had drunken water samples from different treated groups were collected and subjected to total bacterial count to measure the inhibitory capacity of different acidifiers in quail’s drinking water according to (Bolder and Palmu, 1995).B. In vivo evaluation of different acidifiers:Inhibitory capacity of different acidifiers in quail’s after experimental Salmonella infection. Experimental Quails: A total of 200 one-day old Salmonella-free Japanese quail were assigned randomly (40birds/group in two replicates) to one of the following treatment groups: G1 Control group received non-treated drinking tap water, G2, received acetic acid 3ml / liter,G3 received acetic acid 0.5ml/ liter, G4, received organic acid mixture 3ml/liter (the mixture consists of acetic acid, phosphoric acid, lactic acid, fumaric acid, and tartaric acid carried on propylene glycol). While G5, received hydrochloric acid 0.5ml/liter, Treatments were applied in drinking water from day one till the end of the experiment.Quails were raised under optimum environmental temperature and supplied with suitable formulated ration and kept in rooms under complete hygienic conditions in separate caged batteries. Groups were infected with Salmonella Enteritidis. Respective groups were assigned for different acidifiers to ensure their safety and that mortalities were not associated with acidifiers in infected groups.Salmonella Enteritidis infection: A serotype of Salmonella Enteritidis resistance to novobiocin-nalidixic acid (NO 25 ug/ ml, /NA 20 ug /ml) isolated from sick birds and identified by serotyping was selected and maintained on SS agar. Challenge inoculums for intra crop were prepared in sterile phosphate-buffered saline. The viable cell concentration of the inoculums was determined by colony counts on SS agar plates and inoculums contained 1.00.x107 CFU/ml was used to challenge birds at the age of two weeks (Guard et al. 2010).Parameter measured:Clinical signs and mortalities were recorded. At the end of the experimental period ( 28 days post infection) all birds from each group were scarified, autopsied, and examined bacteriologically for re-isolation of SE. from caecum at 7th,14th and 28th days post infection (Quantitative). Liver, spleen, and reproductive organs (testes, ovary and oviduct) were tested qualitative by the end of experiment.Total bacterial and Salmonella Enteritidis count:AT days 7, 21 and 28 days post SE challenge 3 birds from each group were picked up randomly and sacrificed. The caeca aseptically removed and examined for S. Enteritidis quantitatively according to Fukata et al. 1995. Briefly, one gram of caecal content was suspended in a tube containing 9 ml sterile buffered peptone water; the tubes were shaken thoroughly by an electric touch mixer the samples, 0.5 ml from each dilution was withdrawn and spread on: 1) (XLD) contain 25 μg novobiocin/ml and 20 μg nalidixic acid/ml2) Nutrient and MacConkey agar, then plates were incubated at 37°C for 24 hours for total enterobacteriace and total bacterial count of caecal content (CFU/gm).Salmonella Enteritidis colonization in organs (qualitatively):At28th day uniform section of liver, spleen and reproductive organ (testes or ovary and oviduct) were suspended in tetrathionate broth for enriched at 37°C for 24 hours, after enrichment the homogenate (broth) was streaked on XLD plates incubated for 24 hours at 37°C, and examined for typical S E colonies.Statistical analyses:Results are expressed as means ± SEM for each group. Groups were tested for differences by performing the ANOVA and fisher's least protected significance test using IBM SPSS software computer program version 16, NY, USA (Inc., 1989-2010). Differences were considered statistically significant at p<0.05.
3. Results
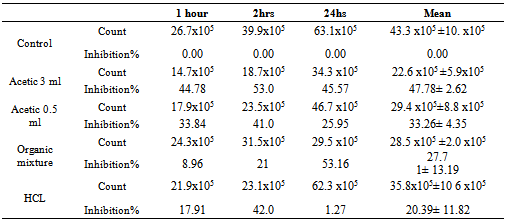
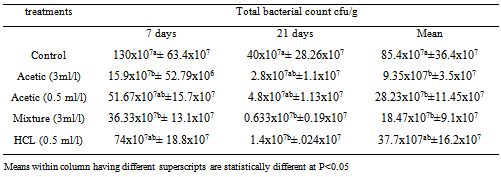
- 1-Time correlated count in culture mediaResults in Table (1) indicated that there was variation between different acidifiers in their effects in terms of time at which they exert their antimicrobial effect also their ability to reduce SE growth in culture medium. HCL was shown to have the lowest time of effect that was mainly observed in an inhibition percent of 17.91 after one hour contact, then increased to reach 42% inhibition, however this effect didn’t last long and percent of inhibition was reduced to 1.27% after 24 hrs contact. On the other hand, acetic acid treatment showed the highest effect on 3 ml/L treated culture as the inhibitory effect increased from 44.78% to 53% in the first and second contact hours, after 24 hrs. The inhibition was reduced to 45.57%. In the same manner acetic acid 0.5 ml/L behaved whereas the inhibition percents were 33.84, 41, and 25.95 % at 1, 3, and 24 hours, respectively. Organic acid mixture showed steady level of inhibition started from 8.96 to 21% and 53.16 % at 1, 2, and 24 hour respectively.A- 2-The inhibitory effect of acidifier for bacterial count in drinking water The results in Table (2) showed that generally there was a reduction in microbial count after 21 days post infection as compared to 7 days post infection in all groups including control non-treated group. Between treatments, a significant (P<0.5) reduction in total bacterial count was observed in both acetic acid (3m/L) and organic acid mixture treated groups at 7 days post infection as compared to control non-treated group, meanwhile there was a trend toward decreasing count in other groups (acetic acid 0.5 ml/l and HCL) as compared to controls. At 21st day post infection there was significant (P<0.5) reduced total bacterial count in HCL and organic mixture treated groups and a trend toward reducing numbers in Acetic acid treated groups as compared to controls. Overall mean of both periods revealed a significant reduction in total bacterial count in acetic acid treated groups as well as organic acid mixture treated group as compared to controls.
|
|
|
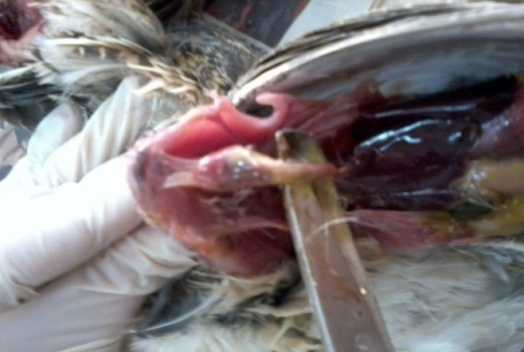 | Figure (1). Post mortem lesions in non- treated controls experimentally infected with Salmonella Enteritidis showing severe serous pericarditis, air sacculitis and severe liver congestion |
 | Figure (2). Post mortum lesions in non- treated control birds eperimentally infected with Salmonella Enteritidis showing severecongestion of ovarian follicles and reduced follicle size |
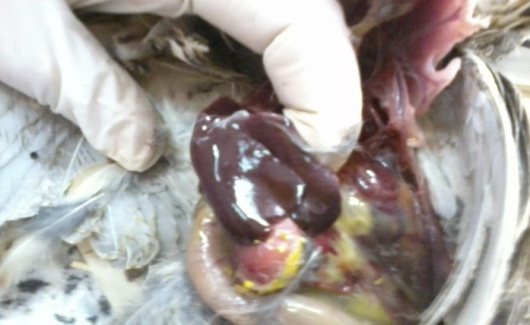 | Figure (3). Post mortem lesions in non- treated control birds experimentally infected with Salmonella Enteritidis showing enlarged friable liver with some pinpoint necrotic foci |
|
|
4. Discussion
- A- In vitro evaluation of different acidifiers.Results concluded the superiority of organic acids over non-organic acid (HCL). Organic acids effects obtained in this study agree with previous reports, as the organic acids may affect the integrity of microbial cell membrane or cell macromolecules or interfere with the nutrient transport and energy metabolism causing the bactericidal effect (Henry et al. 1987). Use of organic acid mixture decreases the total bacterial and Gram negative bacterial counts significantly in the broiler chicken (Gunal et al. 2006). Besides, the butyric acid has been reported to reduce the virulent gene expression and invasiveness in Salmonella Enteritidis, leading to its decreased colonization in the caeca of broiler chicken (Porter and Curtiss 1997 – Van Immerseel et.al. 2004). Also superiority of organic acids in their bactericidal capacity compared to HCL could be explained in the light of time of dissociation, whereas HCL is strong acid, and dissociate quickly than weak organic acids. The antimicrobial effect of an acid depends upon the dissociation constant (pKa) or pH, at which 50% of the total acid is undissociated. The undissociated part of the molecule is related to the antimicrobial effect (Davidson and Taylor, 2007), since the undissociated molecules penetrate into the cells. The activity increases with the chain length, which suggests a direct action of the organic compound itself. Weak acids have higher undissociated portions than strong acids, and they can effectively penetrate through the cytoplasmic membrane (lipid bilayer) of the microorganism (Davidson and Taylor, 2007).B- In vivo evaluation of different acidifiers.Salmonella Enteritidis infection symptoms and Post mortem lesion in quails in our study elaborated the impact of microorganism on birds, our results were confirmed by previous reports of Ezzeldeen and Zouelfakar (2003) Ellakany et al. (2004) and Zohair (2006) reported that treated birds with acidifier could minimize both symptoms and mortalities as well as reduction in microbial shedding and colonization due to drinking water acidification could be a result of antimicrobial effect of different acidifiers and also beneficial effect on cells of gastrointestinal tract. Regarding the antimicrobial effect of different acidifiers on intestinal colonization, there is a discrepancy in the effects of different acidifiers on birds, on one hand, similar effects as compared to our study were observed by Owens et al. (2008) and Pirgozliev et al. (2008) who reported a significantly (p<0.05) reduced total viable coliform numbers in the ileum and caecum of broiler chicken due to organic acid supplementation. Gunal et al. (2006) also reported that the use of organic acid mixture significantly decreased the total bacterial and Gram negative bacterial counts in broiler chicken. On the other hand, many researchers attributed the main effect of organic acids only in the upper part of the digestive tract whereas in a previous study, Mohyla et al. (2007) observed that Salmonella load was significantly reduced in the upper digestive tract but not in the lower digestive tract when acidified sodium cholorite was added to the drinking water at a level of 600 ppm for the last 24 h or 5 d. Similarly, Van Immerseel et al. (2006) reported that acids from feed and water were not effective further down the intestinal tract. According to Hume et al.. 1993; Thompson and Hinton, 1997, most of the short-chain fatty acids (i.e. propionic, formic) used in diets or water are metabolized in the upper gastro-intestinal segments of poultry. Thus, their role in modifying host microflora populations in the lower parts is limited (Józefiak et al. 2010). Recently, some researchers have suggested transport of short-chain fatty acids further down the gastro-intestinal tract by micro-encapsulation in a lipid shell. The protective lipid matrix used for microencapsulation allows organic acids to have an effect all along the gastro-intestinal tract, since they are slowly released during digestion (Fernández-Rubio et al. 2009; Van Immersell et al. 2009). Gheisari et al. (2007) found that supplementation of 0.2% protected organic acids to the diet might improve the proliferation of useful microflora and diminish the population of harmful bacteria in poultry gut contents. Other workers have reported that medium chain fatty acids (C6 to C12; caproic, caprylic, capric and lauric acids) appear to be much more effective against Salmonella than short-chain fatty acids (C≤4; formic, acetic, propionic and butyric acids) (Van Immerseel et al. 2006). In our study, other possible explanations of reducing the shedding and colonization of microorganisms in the lower intestinal tract could be due to continuous administration of acids in drinking water along the experimental period that might causes reduced entry of pathogenic bacteria from the upper parts of GIT (crop) into the intestines of quails and also non-significant reduction of the intestinal pH (Manal et al. 2012). Also, reduced colonization in the internal organs could be as a result of Organic acids have direct stimulatory effect on the gastro-intestinal cell proliferation as was reported by other workers with short chain fatty acids. The short chain fatty acids are believed to increase plasma glucagon-like peptide 2 (GLP-2) and ileal pro-glucagon mRNA, glucose transporter (GLUT2) expression and protein expression, which are all signals which can potentially mediate gut epithelial cell proliferation Tappenden and McBurney (1998). Le Blay et al. (2000) and Fukunaga et al. (2003) also reported that short chain fatty acids can accelerate gut epithelial cell proliferation, thereby increase intestinal tissue weight, which will result in changes of mucosal morphology. Beneficial effect of different acidifiers on epithelial cell proliferation creates the first line of defense against invasion and colonization of pathogenic microorganisms.
5. Conclusions
- Poultry must have a healthy and functional intestinal tract to maintain the excellent feed efficiency that is required by modern production standards. Salmonellosis is considered the major bacterial disease problems in the poultry industry world-wide and these diseases constitute a major public health burden. The economic and public health burden of these diseases have made this topic time demanding. Using acidifiers plays an important role in reducing drinking water microbial contamination and bacterial shedding and colonization through their antibacterial effect and maintaining integrity of cell lining in the gastrointestinal tract that acts as the first line of defense against invasion and colonization of pathogenic microorganisms.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML