-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
p-ISSN: 2325-002X e-ISSN: 2325-0038
2012; 2(4): 23-30
doi:10.5923/j.zoology.20120204.02
The Influence of Palm Oil and Extracted Fishmeal on Growth Performance and Tissue Fatty Acid Composition of Heterobranchus Longifilis Fingerlings
1Department of Animal Science, Landmark University, Omu Aran, Nigeria
2Department of Animal Production, University of Ilorin, Nigeria
Correspondence to: T. O. Babalola, Department of Animal Science, Landmark University, Omu Aran, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Little is known about the effects of the dietary intake of palm oil and extracted fishmeal on growth performance and tissue fatty acid composition of Heterobranchus longifilis. Twenty H. Longifilis fingerlings were allotted for each treatment tanks in triplicate groups at random and were fed ten isonitrogenous and isoenergetic diets during a 12-week feeding trial. Diets were prepared by replacing fish oil with palm oil (PO) at 0, 25, 50, 75 or 100% (of fish oil) in extracted and un-extracted fishmeal-based diets in a 2 x 5 factorial design. Feeding H. longifilisfingerling with various levels of PO did not result in differences in fish growth and feed utilization parameters (P> 0.05). This suggests that complete replacement of FO with PO can support growth comparable to a FO based diet. However, reduced growth and feed utilization parameter values were observed in H. longifilisfingerling fed un-extracted fishmeal-based diets (P < 0.05). Extraction of lipids from fishmeal resulted in lower concentration of tissue eicosapentaenoic acid, docosahexaenoic acid and total n-3 PUFA (p < 0.05). While the tissue concentration of saturated, monounsaturated, n-9, n-6 and 18:2n-6 fatty acids increased in the extracted than the un-extracted groups (P < 0.05). PO and extracted fishmeal resulted in increased index of atherogenicity and index of thrombogenicity of the fillet (P < 0.05), but the value was less than 1.00 considered to be healthy for human consumer. From the results of this study we conclude that H. longifilisfingerling be fed diets containing PO and extracted fishmeal, to enhance fish growth, survival and feed efficiency.
Keywords: Growth Performance, Fatty Acids, Fish Oil, Palm Oil, Heterobranchus Longifilis, Fishmeal
Cite this paper: T. O. Babalola, D. F. Apata, The Influence of Palm Oil and Extracted Fishmeal on Growth Performance and Tissue Fatty Acid Composition of Heterobranchus Longifilis Fingerlings, Research in Zoology , Vol. 2 No. 4, 2012, pp. 23-30. doi: 10.5923/j.zoology.20120204.02.
1. Introduction
- Dietary lipids play an important role as a source of energy for fish growth and as carriers for fat soluble vitamins. Fish oil is widely used in the production of fish feed because of its high content in monounsaturated fatty acids (MUFA) used by fish as metabolic energy source and omega-3 long chain polyunsaturated fatty acids (n-3 lcPUFA) that are essential to biological structure, stress resistance, normal function of cell membranes and development of immunity in fish.[1, 2] The dynamic development of aquaculture with a global average growth rate of 8.8% per year since 1970[3] has resulted in increased demand for fish oil which has reached its limit of sustainability.[4[ On the contrary, production of global vegetable oil has steadily increased reaching a volume of 100 times more than fish oil.[5] Therefore, replacement of fish oil with vegetable oils appears to be a viable option given their availability, low cost and absence of dioxins and pollutants.[6 – 10].Among the vegetable oils, palm oil (PO) is currently the most abundantly produced in the world.[11] PO contains high level of saturated fatty acids especially 16:0 and also contains monounsaturated fatty acids principally made up of 18;1n-9;[12] both classes of fatty acids are good sources of energy for fish.[13] Crude palm oil (CPO) has a deep orange-red colour due to the high content of carotenoids and is a rich source of vitamin E.[14] The use of palm oil at various levels in diets for catfish,[8, 15, 16] Atlantic salmon,[17] and climbing perch[18] resulted in growth and feed utilization efficiency comparable to fish fed with equivalent levels of dietary fish oil. Lim et al.[19] found that the inclusion of at least 8% CPO or RBDPO in African catfish diets significantly improved growth performance, dietary protein retention and fillet vitamin E concentration of this fish. In recent past, CPO and RBDPO have been evaluated as dietary lipids for fish and this was done in the presence of residual fish oil from the fishmeal component in the experimental diets.[16 - 19, 20] However, fishmeal contains approximately 10% fish oil as purchased, which might provide sufficient HUFA to meet the essential fatty acid requirement of H. longifilis and obviate the effects of the lipid of interest.In the present study, the nutritive value of palm oil as a lipid source for African catfish H. longifilis using an extracted fishmeal–based diet was evaluated. The study was designed to eliminate any potential confounding effects of the residual fish oil found in fishmeal as used in previous study.[21] The principal objective of this study was to investigate the effect of extracted fishmeal and palm oil on growth, feed utilization efficiency, fillet and liver fatty acid composition of Heterobranchus longifilis fingerlings.
2. Materials and Methods
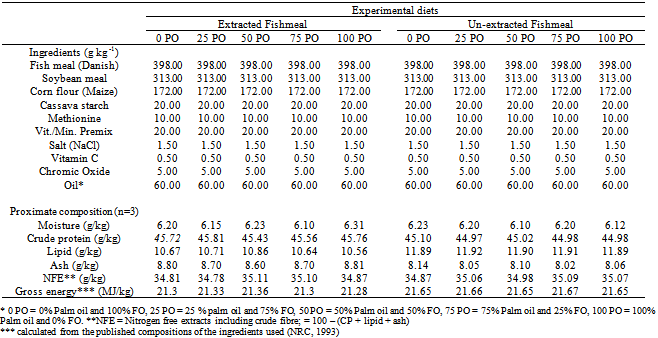
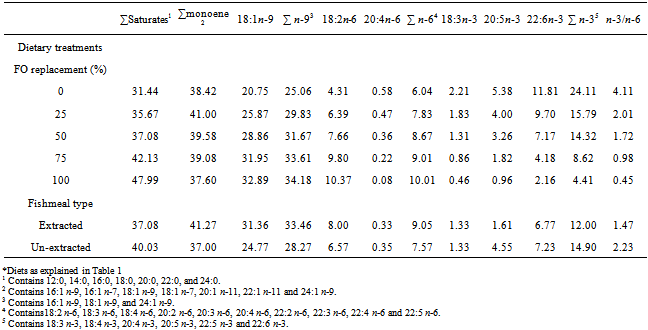
- Experimental dietsPalm oil was selected and used for this study based on the superior performance exhibited by H. longifilis fed palm oil containing diet in the previous study.[22] The Danish fish meal used as the primary protein source in the test diets was exhaustively extracted with hexane to reduce the residual lipid to trace amounts (about 1.0%) before inclusion in the diets. Ten isonitrogenous and isolipidic experimental diets (Table 1) were formulated by replacing fish oil with palm oil at 0, 25, 50, 75 or 100% (of fish oil) in extracted and un-extracted fishmeal-based diets.The diets were formulated to contain similar contents of total fat, protein and energy. This was confirmed by chemical analysis (Table 1). The difference in the diets was the levels of PO and the fishmeal type, leading to variations in the fatty acid concentration of the diets (Table 2). This study was carried out using thirty circular bowls capable of holding 62 litres of water in a 2 x 5 factorial design. Each bowl was filled to a depth of 35cm with water from storage reservoir. Six hundred H. longifilis were randomly stocked in 30 circular bowls at 20 fingerlings per tank; 60 fingerlings per treatment, experimental diets were fed to triplicate groups of fish twice a day to apparent satiation at 09:00 and 16:00 respectively over a period of twelve weeks. One hour after feeding, uneaten feed was siphoned, dried to constant weight at 700C and reweighed. Total fish weight in each tank was determined every two weeks to check their growth. Feeding was stopped 24 h prior weighing.Sample collection and analysisDiets were analysed for proximate composition using standard methods.[23] Moisture was determined gravimetrically after drying in oven at 105℃ for 24 h; ash by incineration in a muffle furnace at 4500C for 16 h; protein (N x 6.25) by the Kjeldahl method after acid digestion and lipids by petroleum ether extraction. At the end of the trial, H. longifilis was fasted for 24 h and anesthetized with solution of methane sulphonate (MS222).[24] The fish were then dissected and their liver removed and weighed for the determination of hepatosomatic index (HSI). Fatty acid analysis was performed on three samples of each experimental diet and pooled fillet and liver samples for each experimental treatment. The extraction of total lipids and preparation of fatty acid methyl esters was performed according to Sukhija and Palmquist.[25] Fatty acids methyl esters were separated by GC on a Varian Model 3300 gas chromatograph equipped with flame ionisation detector. A flexible fused silica Megabore column (30 m X 0.32 mm, 1 µm film thickness) with bonded stationary phase of CP-WAX was employed. Helium was used as the carrier gas at 1.0 ml/min, inlet pressure 12 psi. Injector and the detector temperatures were 250 and 300℃, respectively. The column temperature was programmed to be initially 140℃ for 5 min and then to increase at a rate of 3℃ to a final temperature of 240℃. Fatty acids methyl esters were identified in comparison to an external standard (SupelcoTM 37 component FAME Mix).
|
|
3. Results
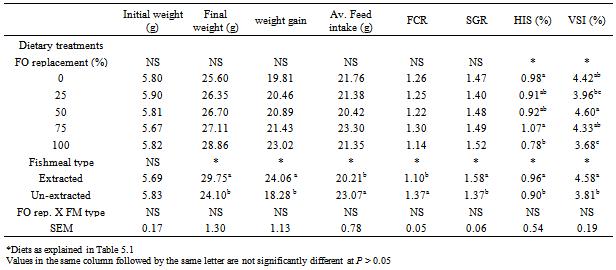
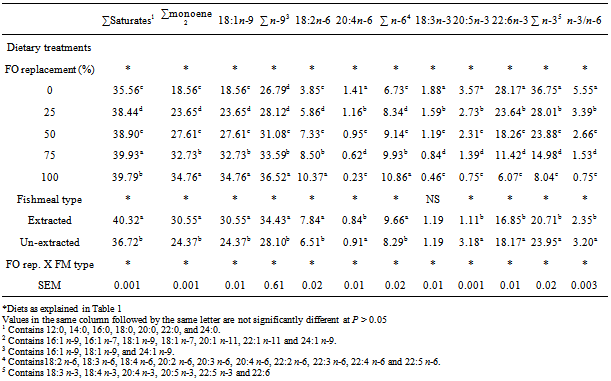
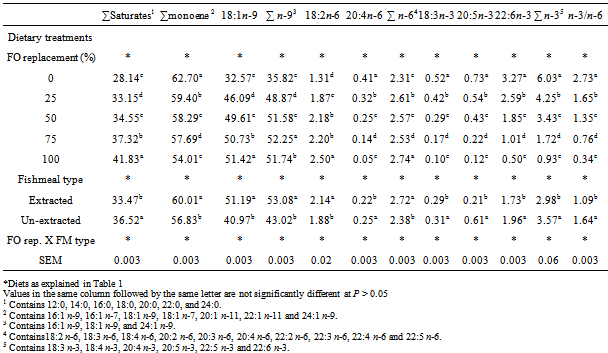
- PerformanceThere was no significant difference in the initial weight of H. longifilis fingerlings (P > 0.05) (Table 3). The mean final weights of H. longifilis fingerling fed all levels of palm oil substitution for fish oil were not significantly different. All group of fish increased in weight by around fourfold (400%) and specific growth rates (SGR) varied between 1.40 and 1.52. Similar trends were evident for feed utilization parameters [average feed intake (AVFI) and feed conversion ratio (FCR)]. There were no significant differences between AVFI or FCR for all fish fed PO diets. The lowest FCR (1.14) was observed for 6% PO (100% FO replacement) treatment, while AVFI ranged from 20.42 (3% PO) to 23.30 (4.5% PO). However, significant (P < 0.05) differences were observed between fish fed extracted and un-extracted fishmeal diets. The un-extracted fish group had lower values for final weight, weight gain, average feed intake, feed conversion ratio and specific growth rates (SGR) than those fed extracted fishmeal diet.Viscerosomatic and hepatosomatic indices were both significantly higher in fish fed diets containing 0, and 4.5% PO than 6% PO diet. However, hepatosomatic index (HSI) of fish fed 0%, 1.5% and 3% PO were similar. Fish fed diet in which PO replaced 100% FO (6% PO) had the lowest liver weight. Viscerosomatic index (VSI) of fish fed 25% and 100% PO were similar and significantly (P < 0.05) decreased compared with fish fed 0%, 50% or 75% PO. Extraction of lipids from fishmeal used for this study had significant (P < 0.05) effects on HSI and VSI of fish. HSI and VSI of fish fed un-extracted fishmeal-based diets were significantly (P < 0.05) higher than those fed extracted fishmeal-based diets. There was no interaction between PO substitution and fishmeal type on any of the growth parameters measured.Fillet fatty acid compositionThe fatty acid composition of the fillet is shown in Table 4. There were significant interaction between dietary PO and fishmeal type on fatty acid composition of fillets. Thus 18:3n-3, 20:5n-3 and 22:6n-3 of the fish fed the control diet (6% FO) were significantly higher than those fed diets in which PO replaced 25, 50, 75 and 100% of the FO in the control diet (P < 0.05). Fish fed diets with 6% PO (100% replacement) had the lowest concentrations of these fatty acids. However, the concentration of monounsaturated, sum n-9 and sum n-6 fatty acids were significantly higher in the fillet of fish fed diet in which FO was replaced 100% with PO. Fish fed diet in which 75% FO was replaced with PO had the highest concentration of saturated fatty acid, which was also significantly (P < 0.05) higher than those of fish fed other diets. Fish fed the extracted fish meal-based diets exhibited higher (P < 0.05) concentration of saturated, monounsaturated, sum n-9, sum n-6 and 18:2n-6 fatty acids in the fillet than the un-extracted group. On the other hand the concentrations of sum n-3, 20:5n-3 and 22:6n-3 were significantly (P < 0.05) reduced in the fillets of fish fed extracted fish meal diets. Significant (P < 0.05) effects of FO substitution and fish meal type interaction were observed on all fillet fatty acids measured. Fillet sum saturated, sum monounsaturated, 18:1n-9, sum n-9, 18:2n-6 and sum n-6 fatty acids were significantly (P < 0.05) higher in fish fed extracted fish meal and 100% FO replacement while un-extracted fish meal and 0% PO fed fish had least concentrations of these fatty acids in their fillet. On the other hand fish fed 0% PO and un-extracted fish meal diets had the highest concentration of 20:4n-6, sum n-3, 18:3n-3, 20:5n-3, 22:6n-3 fatty acids and n-6:n-3 fatty acid ratio. The least concentration occurred in the fillet of fish fed extracted fish meal and 100% FO replacement.
|
|
|
|
4. Discussion
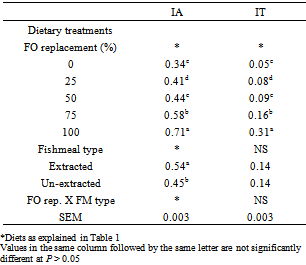
- The central issue in this trial is the feasibility of replacing FO with PO. The results shows that substantial part of marine FO can be replaced with vegetable alternatives especially PO. PO can meet the energy requirement of fish by providing easily oxidised fatty acids especially 18:1n-9 and simultaneously generate improved fillet quality by the deposition of tocopherols and tocotrienols in fish fillets.[28] These also add value to the product, the health benefit of tocotrienols in human diet has been shown to include the prevention of cardiovascular diseases and cancer.[28] The accumulation of palm vitamin E in fish flesh would also slow down the process of oxidation thereby maintaining the quality of the flesh for longer period. This study demonstrated the absence of a significant difference in growth rate or feed conversion ratio in H. longifilis fed diets containing PO ranging from 25% to 100% replacement for FO and those fed FO (0% PO). This suggests that complete replacement of FO with PO can support growth comparable to a FO based diet. The results compare favourably with the reports of Torstensen et al.[17], Bell et al.[13] in which the growth and feed conversion ratio were similar at all levels of PO replacement for FO in the diet of Atlantic salmon.The suitability of palm oil as a dietary lipid and energy source has been demonstrated for various tropical fish.[9, 13, 16] H. longifilis, like other warmwater fish, are more inclined to require greater amounts of n-6 fatty acids compared to n-3 fatty acids for maximal growth.[29] High levels of n-3 PUFA have been reported to depress the growth of tilapia,[30, 31] channel catfish, Ictalurus punctatus[32] and African catfish Clarias gariepinus.[8] However, Chou et al.[33] reported no growth depression in hybrid tilapia fed semi purified diets with graded levels of CLO up to 5%. In this study, since H. longifilis grew better when fed with PO diets (high in n-6 PUFA) compared to fish fed with CLO diets (high in n-3 PUFA), this results suggest that H. longifilis require n-6 fatty acids for growth. The reduced growth observed in fish fed with the un-extracted fish meal diet might be associated with the higher n-3 PUFA concentrations in the fish oil.The fatty acid compositions of fillet lipids were closely influenced by dietary fatty acid input. However, while dietary fatty acids are closely related to fatty acids deposited in the fillet, specific fatty acids are selectively retained or utilized. There was selective deposition and retention of DHA, since fillet DHA concentrations were always higher than diet concentrations. This fact has also been observed in Atlantic salmon,[13, 34, 35] rainbow trout,[6] African catfish[8] and turbot.[36, 37] The possible mechanisms underlying this selective deposition include the high specificity of fatty acyl transferases for DHA and the relative resistance of DHA to β- oxidation stemming from the complex catabolic pathway for this HUFA.[34]However, monounsaturated fatty acids, LA, LNA and EPA were selectively utilized in flesh, when present at high concentrations in the diet, whereby dietary concentrations were always higher than flesh concentrations.[6, 8, 13, 34 – 37], These data suggest that the monoenes, as well as LA and LNA, are readily oxidized when present at high concentrations. However, the levels of LA in H. longifilis flesh remained significantly higher in fish fed diet containing 100% PO and extracted fish meal than in FO (0% PO and un-extracted fish meal) fish.The liver fatty acid compositions were closely related to diet compositions as observed in the fillet. In comparison to fillet fatty acids, there was selective retention of monounsaturated fatty acids in the liver, but no selective retention of the n-3 HUFA, EPA and DHA. However, as was seen in fillet, LA and LNA were not selected for deposition in liver lipids. Lipid storage depots in fish are used to store fat that are not required immediately for physiological functions such as synthesis of cell membrane phospholipids.[38] In addition, storage lipids also act as a reserve of metabolic energy that may be used during times of low feed intake and to provide fatty acids for reproductive functions, including energy production.[38] For this reason the retention of monounsaturated fatty acids may be related to energy storage, as these fatty acids are preferred substrates for β-oxidation in fish.[17, 39] H. longifilis displayed high bioconversion efficiency of n-3 fatty acids, which results in a higher concentration of DHA in the fillet of the fish compared to the level of this fatty acid in their diet. The IA and IT take the interactions among different fatty acids into account, allowing an integrated assessment of dietary lipid on human coronary health.[26] Higher values of IT and IA (>1.0) are detrimental to human health.[40] There were statistically significant differences in the IA of fillet from H. longifilis fed diets with different PO replacement levels and extracted and un-extracted fish meal, but the values do not exceed 0.71 and 0.54 for fish fed the various levels of PO and extracted and un-extracted fish meal-based diets, respectively. Therefore, the differences in potential health impact of fillets from H. longifilis fed the experimental diets appear to be minor. Similar results were obtained in largemouth bass fed diet with fish oil compared to fish fed diets with canola, chicken fat or combination of chicken fat and menhaden fish oil.[41]From the results of this study, it is logical to conclude that the growth performance, survival and feed efficiencies were significantly higher in fish fed diets containing PO and extracted fishmeal than in the fish fed FO. However, fish’s fatty acid composition especially n-3 HUFA were reduced in fish fed higher PO levels and extracted fishmeal based diets, which may subsequently affect the quality of the fish in terms of human n-3 and n-6 fatty acid requirement. To address this, further studies is therefore needed to reverse this change in fatty acid compositions by evaluating the effects of adding combinations of fish oil and vegetable oils to the diet of H. longifilis on growth, lipogenesis and tissue fatty acid composition.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML