-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Zoology
p-ISSN: 2325-002X e-ISSN: 2325-0038
2012; 2(4): 19-22
doi:10.5923/j.zoology.20120204.01
Acute Toxicity of Nickel and Cadmium to the Cichlid Fish, Oreochromis mossambicus (Peters)
Dorey Benjamin, A. J. Thatheyus
P.G. & Research Department of Zoology, The American College, Madurai, 625 002, Tamil Nadu, India
Correspondence to: A. J. Thatheyus, P.G. & Research Department of Zoology, The American College, Madurai, 625 002, Tamil Nadu, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
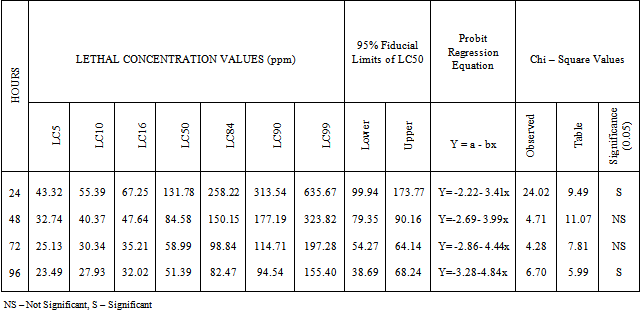
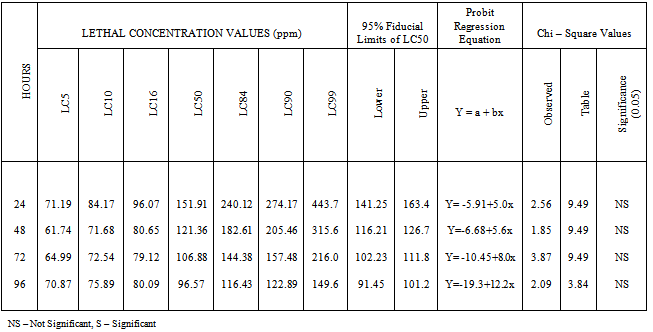
Both nickel and cadmium are used in various industries and hence their effluents contain these two metals. As they are toxic and alter the physiological and biochemical parameters of fish,acute toxicity of nickel and cadmium to the fresh water fish Oreochromis mossambicus was determined using static bioassays. Fish were exposed to selected concentration of nickel and cadmium separately and the mortality data were determined after 24, 48, 72 and 96 hours. LC50 values for 24, 48, 72 and 96 hours were calculated with the 95% fiducial limits through probit analysis and goodness of fit was evaluated with Chi – square tests. The 24, 48, 72 and 96 hours LC50 values of nickel to the fish were 131.78, 84.58, 58.19 and 51.39 ppm respectively. For cadmium the 24, 48, 72 and 96 h LC50 values were 151.91, 121.36, 106.88 and 96.57 ppm respectively. Among the two heavy metals tested, nickel is more toxic to the fish than cadmium. These LC50 values will help to fix the sub-lethal concentrations and study the sub- toxic effects of these two metals in the selected fish.
Keywords: Acute Toxicity, Nickel, Cadmium, Oreochromis Mossambicus
Cite this paper: Dorey Benjamin, A. J. Thatheyus, Acute Toxicity of Nickel and Cadmium to the Cichlid Fish, Oreochromis mossambicus (Peters), Research in Zoology , Vol. 2 No. 4, 2012, pp. 19-22. doi: 10.5923/j.zoology.20120204.01.
Article Outline
1. Introduction
- The impacts of industrialization and exponentially growing population, contamination of air, water, soil and food have become a threat to the continued existence of the living communities of the ecosystem and may threaten the very survival of the human race. The indiscriminate dumping of untreated wastes into aquatic environments brings about physical, chemical and biological deterioration of such water bodies, when these discharges are beyond their self purifying capacity. This will endanger the aquatic organisms and impair the beneficial uses of water[1]. Heavy metals pose serious environmental problems as they are the major constituents of industrial effluents. The contamination of aquatic environment with heavy metals is of serious concern as they cannot be removed or nonbiodegradable. They pose a severe threat to aquatic organisms[2]. Nickel and cadmium are employed in several industries and they exert a variety of bad effects[3]. Acute toxicity of nickel to various fish species is already reported[4, 5, 6, 7, 8, 9, 10, 11, 12, 13, and 14]. Reports are also available on the toxicity of cadmium to fish[15, 16, 17, 18, 19 and 20]. The test organism chosen for the present study, Oreochromis mossambicus is available throughtout the year, cheaper and protein rich food[21]. Assessing the acute toxicity of the heavy metals to fish will help to design environmental monitoring strategies and ecosystem conservation measures. Hence in the present work an attempt has been made to study the acute toxicity of nickel and cadmium to O.mossambicus employing static bioassays. In the present study the LC5, 10, 16, 50, 84, 90, 99 values are derived along with upper and lower 95 % fiducial limits of LC50. As a special the probit regression equations for 24, 48, 72 and 96 hour exposure for the two metals were subjected to analyse the goodness of fit using chi – square testes.
2. Materials and Methods
- Fish weighing about 15 g were procured from a local fish farm and were acclimatized to the laboratory conditions. The stock solutions of nickel and chromium were prepared separately and the desired concentrations were prepared by adopting the dilution techniques. Based on the progressive bisection of intervals on a logarithmic scale, log concentrations were selected as the experimental concentrations. These concentrations were fixed after conducting the exploratory bioassays. Feeding was stopped two days before the fish were subjected to experiment to avoid change in toxicity of metals due to excretory products. After the addition of toxicant into the test tank having 10 fish, mortality was recorded after 24, 48, 72 and 96 hours[22]. The mortality data were subjected to weighted probit analysis[23]. The median lethal concentration (LC50) values for 24, 48, 72 and 96 hours were calculated with the 95% fiducial limits. Goodness of fit was evaluated with Chi-square tests[24].
3. Results
- Table 1 reveals the results of weighted probit analysis for nickel from percent response of O.mossambicus after 24, 48, 72 and 96 hours. LC50 values, 95% fiducial limits and fitted regression equation for each hour have been given. The chi-square test shows that two values (24 and 96h) are significant and the other two (48 and 72h) are not significant. There is a decline in LC50 value from 24h towards 96h as 131.78, 84.58, 51.39 and 58.99 ppm. Decrease in LC50 value is noted with the increase in exposure period. The difference between the LC50 values of 72 and 96h is low and the difference between 24h and 48h is high. For cadmium, there is a decline in LC50 value from 24h to 96h as 131.78, 84.58, 51.39 and 58.99 ppm. Decrease in LC50 value is noted with the increase in exposure period. The difference between the LC50 values of 72 and 96h is low and the difference between 24h and 48h is high. For cadmium, there is a decline in LC50 value from 24h to 96h as 151.91, 121.36, 106.88 and 96.57 ppm (Table 2). The difference between the LC50 values of 72 and 96h is high whereas it is low in the case of 48 and 72h. Among the two metals tested, nickel is more toxic to O.mossambicus than cadmium.
|
|
4. Discussion
- Acute toxicity tests are useful in providing rapid estimates of the concentrations of toxicants that cause direct irreversible harm to the test organism[25]. Based on the results nickel is found to be more toxic to the fish than cadmium. 96h LC50 values of nickel of striped bass, rainbow trout[11], fathead minnow and bluegill[6], common carp[14] were 3.9, 15.0, 4.58, 5.18 and 47.58 ppm respectively. Hence, it can be deduced that O.mossambicus is more resistant to nickel when compared to other fish. Certain fish like Channa punctatus[26], Mystus vittauts[9], Notopterus notopterus[14] exhibited higher LC50 values than O.mossambicus. O.mossambicus is also not so sensitive to cadmium than marine crustaceans which may be due to high content of free nickel in soft than salt water[27]. These test results will help to fix the quality criteria of effluents from industries which are discharged into the environment
5. Conclusions
- Effluents from industries generally contain heavy metals as major constituents and among them nickel and cadmium are important. Hence toxicity of this two metals to O.mossambicus was determined using static bioassays following probit analysis. Based on the acute toxicity test results it can be concluded that nickel is more toxic to the fish than cadmium. The selected fish O.mossambicus is more resistant to both nickel and cadmium compared to other fresh water fish. The 96 hour LC50 value of nickel and cadmium to the fish are 51.39 and 96.57ppm respectively. Thus this data will help to proceed for studies related to sub-lethal toxicity of these two metals and design pollution monitoring strategies.
ACKNOWLEDGEMENTS
- The authors thank the authorities of The American College, Madurai for the financial assistance and facilities.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML